Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0267)
| Name |
Isorhynchophylline
|
||||
|---|---|---|---|---|---|
| Synonyms |
Isorhynchophylline; 6859-01-4; Isorhyncophylline; 7-Isorhyncophylline; Isorhychophylline; 7F4P99KHLJ; CHEBI:70071; UNII-7F4P99KHLJ; methyl (E)-2-[(3S,6'R,7'S,8'aS)-6'-ethyl-2-oxospiro[1H-indole-3,1'-3,5,6,7,8,8a-hexahydro-2H-indolizine]-7'-yl]-3-methoxyprop-2-enoate; (16E,20-alpha)-16,17-Didehydro-17-methoxy-2-oxo-corynoxan-16-carboxylic acid; Corynoxan-16-carboxylic acid, 16,17-didehydro-17-methoxy-2-oxo-, methyl ester, (16E,20-alpha)-; Rel-methyl (E)-2-((3S,6'R,7'S,8a'S)-6'-ethyl-2-oxo-2',3',6',7',8',8a'-hexahydro-5'H-spiro[indoline-3,1'-indolizin]-7'-yl)-3-methoxyacrylate; 39032-62-7; IsoRhy; MLS000728608; CHEMBL480521; SCHEMBL23259272; HMS2267O23; HMS3886F22; HY-N0766; BDBM50531282; BP0799; s9310; AKOS025402306; AC-7996; CCG-268459; CS-3805; AS-75287; SMR000470794; C16980; Q-100013; ISORHYNCHOPHYLLINE (CONSTITUENT OF CAT'S CLAW); Q27138409; ISORHYNCHOPHYLLINE (CONSTITUENT OF CAT'S CLAW) [DSC]; CORYNOXAN-16-CARBOXYLIC ACID, 16,17-DIDEHYDRO-17-METHOXY-2-OXO-, METHYL ESTER, (16E,20.ALPHA.)-; CORYNOXAN-16-CARBOXYLIC ACID, 16,17-DIDEHYDRO-17-METHOXY-2-OXO-, METHYL ESTER, (16E,20alpha)-; methyl (2E)-2-[(3S,6'R,7'S,8'aS)-6'-ethyl-2-oxo-1,2,3',5',6',7',8',8'a-octahydro-2'H-spiro[indole-3,1'-indolizin]-7'-yl]-3-methoxyprop-2-enoate; SPIRO(3H-INDOLE-3,1'(5'H)-INDOLIZINE)-7'-ACETIC ACID, 6'-ETHYL-1,2,2',3',6',7',8',8'A-OCTAHYDRO-.ALPHA.-(METHOXYMETHYLENE)-2-OXO-, METHYL ESTER, (.ALPHA.E,1'S,6'R,7'S,8'AS)-; SPIRO(3H-INDOLE-3,1'(5'H)-INDOLIZINE)-7'-ACETIC ACID, 6'-ETHYL-1,2,2',3',6',7',8',8'A-OCTAHYDRO-alpha-(METHOXYMETHYLENE)-2-OXO-, METHYL ESTER, (alphaE,1'S,6'R,7'S,8'AS)-; Spiro[3H-indole-3,1'(5'H)-indolizine]-7'-acetic acid,6'-ethyl-1,2,2',3',6',7',8',8'a-octahydro-a-(methoxymethylene)-2-oxo-,methyl ester, (aE,1'S,6'R,7'S,8'aS)-
Click to Show/Hide
|
||||
| Structure |
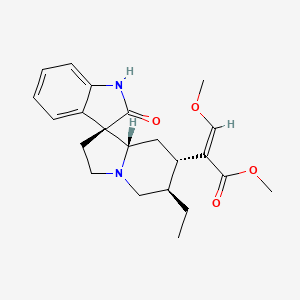 |
||||
| Formula |
C22H28N2O4
|
||||
| IUPAC Name |
methyl (E)-2-[(3S,6'R,7'S,8'aS)-6'-ethyl-2-oxospiro[1H-indole-3,1'-3,5,6,7,8,8a-hexahydro-2H-indolizine]-7'-yl]-3-methoxyprop-2-enoate
|
||||
| Canonical SMILES |
CCC1CN2CCC3(C2CC1C(=COC)C(=O)OC)C4=CC=CC=C4NC3=O
|
||||
| InChI |
InChI=1S/C22H28N2O4/c1-4-14-12-24-10-9-22(17-7-5-6-8-18(17)23-21(22)26)19(24)11-15(14)16(13-27-2)20(25)28-3/h5-8,13-15,19H,4,9-12H2,1-3H3,(H,23,26)/b16-13+/t14-,15-,19-,22-/m0/s1
|
||||
| InChIKey |
DAXYUDFNWXHGBE-VKCGGMIFSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Cystine/glutamate transporter (SLC7A11)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Responsed Regulator | Cellular tumor antigen p53 (TP53) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| p53 signaling pathway | hsa04115 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (SD rats, weighing 250-300 g) aged 11-12 weeks were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All 96 rats were randomly divided into four groups of 24 rats each: Sham group, Sham + IRN (30 mg/Kg) group, ICH group, and ICH + IRN (30 mg/Kg) group. The rats in sham group were injected with PBS solution, and the Sham + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection) after the sham operation. After ICH, the rats in ICH group were injected with PBS solution, and the ICH + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection).
Click to Show/Hide
|
||||
| Response regulation | Isorhynchophylline (IRN) decreased ferroptosis and lipid ROS level, upregulated the expression of miR-122-5p and SLC7A11 mRNA, and inhibited TP53 expression. In conclusion, IRN protects neurocyte from intracerebral hemorrhage (ICH)-induced ferroptosis via miR-122-5p/TP53/SLC7A11 pathway, which may provide a potential therapeutic mechanism for ICH. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Responsed Regulator | hsa-miR-122-5p (miRNA) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| p53 signaling pathway | hsa04115 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (SD rats, weighing 250-300 g) aged 11-12 weeks were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All 96 rats were randomly divided into four groups of 24 rats each: Sham group, Sham + IRN (30 mg/Kg) group, ICH group, and ICH + IRN (30 mg/Kg) group. The rats in sham group were injected with PBS solution, and the Sham + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection) after the sham operation. After ICH, the rats in ICH group were injected with PBS solution, and the ICH + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection).
Click to Show/Hide
|
||||
| Response regulation | Isorhynchophylline (IRN) decreased ferroptosis and lipid ROS level, upregulated the expression of miR-122-5p and SLC7A11 mRNA, and inhibited TP53 expression. In conclusion, IRN protects neurocyte from intracerebral hemorrhage (ICH)-induced ferroptosis via miR-122-5p/TP53/SLC7A11 pathway, which may provide a potential therapeutic mechanism for ICH. | ||||
