Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0249)
| Name |
Atranorin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Atranorin; 479-20-9; Atranoric acid; Atranorine; Parmelin; Usnarin; Antranoric acid; Parmelin acid; Usnarin acid; 3-Hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl 3-formyl-2,4-dihydroxy-6-methylbenzoate; NSC 249980; NSC 685591; NSC-685591; 450U2VJ2VG; CHEMBL173395; Benzoic acid,3-formyl-2,4-dihydroxy-6-methyl-,3-hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl ester; NSC685591; NSC-249980; 3-formyl-2,4-dihydroxy-6-methylbenzoic acid 3-hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl ester; Benzoic acid, 3-formyl-2,4-dihydroxy-6-methyl-, 3-hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl ester; EINECS 207-527-7; UNII-450U2VJ2VG; Benzoic acid,4-dihydroxy-6-methyl-, 3-hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl ester; Isophthalaldehydic acid,4-dihydroxy-6-methyl-, 4-ester with methyl 3,6-dimethyl-.beta.-resorcylate; Spectrum_000143; SpecPlus_000239; ATRANORIN [MI]; Spectrum2_001749; Spectrum3_001716; Spectrum4_001700; Spectrum5_000380; (3-hydroxy-4-methoxycarbonyl-2,5-dimethylphenyl) 3-formyl-2,4-dihydroxy-6-methylbenzoate; BSPBio_003332; KBioGR_002000; KBioSS_000623; SPECTRUM200034; DivK1c_006335; SPBio_001858; SCHEMBL1370191; KBio1_001279; KBio2_000623; KBio2_003191; KBio2_005759; KBio3_002552; DTXSID10197319; CHEBI:144119; YLOYKYXNDHOHHT-UHFFFAOYSA-N; HY-N2907; NSC87512; BDBM50056919; CCG-38801; MFCD00016597; NSC-87512; NSC249980; AKOS024319117; SDCCGMLS-0066426.P001; NCGC00095465-01; NCGC00095465-02; Isophthalaldehydic acid, 2,4-dihydroxy-6-methyl-, 4-ester with methyl 3,6-dimethyl-beta-resorcylate; MS-26044; CS-0023502; FT-0769622; SR-05000002629; SR-05000002629-1; Q27258788; (3-hydroxy-4-methoxycarbonyl-2,5-dimethyl-phenyl) 3-formyl-2,4-dihydroxy-6-methyl-benzoate; 3-Hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl 3-formyl-2,4-dihydroxy-6-methylbenzoate #; Methyl 4-(3-formyl-2,4-dihydroxy-6-methyl-benzoyloxy)-2-hydroxy- 3,6-dimethyl-benzoate; Isophthalaldehydic acid, 2,4-dihydroxy-6-methyl-, 4-ester with methyl 3,6-dimethyl-.beta.-resorcylate; methyl 1-(3-formyl-2,4-dihydroxy-6-methylphenylcarbonyloxy)-3-hydroxy-2,5-dimethyl-4-benzenecarboxylate
Click to Show/Hide
|
||||
| Structure |
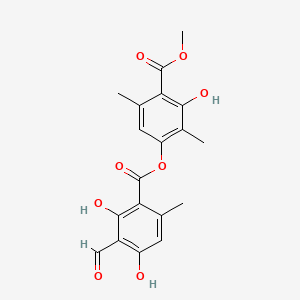 |
||||
| Formula |
C19H18O8
|
||||
| IUPAC Name |
(3-hydroxy-4-methoxycarbonyl-2,5-dimethylphenyl) 3-formyl-2,4-dihydroxy-6-methylbenzoate
|
||||
| Canonical SMILES |
CC1=CC(=C(C(=C1C(=O)OC2=C(C(=C(C(=C2)C)C(=O)OC)O)C)O)C=O)O
|
||||
| InChI |
InChI=1S/C19H18O8/c1-8-5-12(21)11(7-20)17(23)15(8)19(25)27-13-6-9(2)14(18(24)26-4)16(22)10(13)3/h5-7,21-23H,1-4H3
|
||||
| InChIKey |
YLOYKYXNDHOHHT-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hGCCs (Gastric cancer cells) | ||||
| In Vivo Model |
NOD-scid mice (NOD.CB17-Prkdcscid/NcrCrl) aged 6-7 weeks and weighing 20-22 g were used in the experiment. The animal study was performed at the Shanghai University of Traditional Chinese Medicine with approval from the Institutional Animal Care and Use Committee in accordance with the institutional guidelines. All mice were randomly divided into two groups, and each group consisted of four mice. In experimental group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul Atranorin@SPION (10 mg/kg) every 2 days. In control group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul SPION (10 mg/kg) alone every 2 days. After 2 months, the mice were sacrificed, and their tumors were excised.
Click to Show/Hide
|
||||
| Response regulation | Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in gastric cancer cells. This study revealed the molecular biological mechanism by which Atranorin@SPION inhibit the in vitro and in vivo activity of GCSCs, that is, Atranorin@SPION reduced the expression of members of the Xc-/GPX4 axis and reduced their mRNA 5-hydroxymethylcytidine modification, finally induced ferroptosis of GCSCs. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hGCCs (Gastric cancer cells) | ||||
| In Vivo Model |
NOD-scid mice (NOD.CB17-Prkdcscid/NcrCrl) aged 6-7 weeks and weighing 20-22 g were used in the experiment. The animal study was performed at the Shanghai University of Traditional Chinese Medicine with approval from the Institutional Animal Care and Use Committee in accordance with the institutional guidelines. All mice were randomly divided into two groups, and each group consisted of four mice. In experimental group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul Atranorin@SPION (10 mg/kg) every 2 days. In control group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul SPION (10 mg/kg) alone every 2 days. After 2 months, the mice were sacrificed, and their tumors were excised.
Click to Show/Hide
|
||||
| Response regulation | Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in gastric cancer cells. This study revealed the molecular biological mechanism by which Atranorin@SPION inhibit the in vitro and in vivo activity of GCSCs, that is, Atranorin@SPION reduced the expression of members of the Xc-/GPX4 axis and reduced their mRNA 5-hydroxymethylcytidine modification, finally induced ferroptosis of GCSCs. | ||||
