Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0075)
| Name |
Kaempferol
|
||||
|---|---|---|---|---|---|
| Synonyms |
kaempferol; 520-18-3; Robigenin; Kaempherol; Kempferol; Populnetin; Rhamnolutein; Trifolitin; Swartziol; Pelargidenolon; Rhamnolutin; 3,4',5,7-Tetrahydroxyflavone; Indigo Yellow; Kampherol; Campherol; Kampferol; Nimbecetin; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; Kaemferol; 5,7,4'-Trihydroxyflavonol; Pelargidenolon 1497; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; C.I. 75640; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one; Pelargidenon; Kampcetin; CCRIS 41; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-; Flavone, 3,4',5,7-tetrahydroxy-; NSC 407289; NSC 656277; EINECS 208-287-6; Kempferol;Robigenin; NSC-407289; NSC-656277; UNII-731P2LE49E; BRN 0304401; 3,5,7,4'-Tetrahydroxyflavone; DTXSID7020768; CHEBI:28499; AI3-36096; HSDB 7703; 731P2LE49E; 3'-DEOXYQUERCETIN; MFCD00016938; CHEMBL150; DTXCID30768; 5-18-05-00251 (Beilstein Handbook Reference); NSC656277; CAS-520-18-3; CI 75640; KAEMPFEROL (IARC); KAEMPFEROL [IARC]; SMR000112585; 4det; Kaempferol,(S); KAEMPFEROL [MI]; 5,4'-Trihydroxyflavonol; Prestwick0_001098; Prestwick1_001098; Prestwick2_001098; Prestwick3_001098; KAEMPFEROL [HSDB]; KAEMPFEROL [INCI]; 3,5,7-Tetrahydroxyflavone; KAEMPFEROL [USP-RS]; BIDD:PXR0073; Oprea1_650954; SCHEMBL18817; BSPBio_001176; MLS000697730; MLS001055391; MLS001074884; MLS006010737; BIDD:ER0134; SPBio_003058; Kaempferol, analytical standard; BDBM7462; BPBio1_001294; MEGxp0_001283; Flavone,4',5,7-tetrahydroxy-; ACon1_001867; cid_5280863; GTPL11052; CHEBI: 28499; HMS1571K18; HMS2098K18; HMS2267I09; HMS3414C03; HMS3656M03; HMS3678C03; HMS3884B13; 4H-1-Benzopyran-4-one,3,5,7-trihydroxy-2-(4-hydroxyphenyl)-; Kaempferol, >=97.0% (HPLC); TNP00039; Tox21_201165; Tox21_303363; AC-544; HSCI1_000027; LMPK12110003; NSC407289; s2314; AKOS015895240; Kaempferol, >=90% (HPLC), powder; CCG-202823; CS-1273; DB01852; GS-3570; NCGC00016480-01; NCGC00016480-02; NCGC00016480-03; NCGC00016480-04; NCGC00016480-05; NCGC00016480-06; NCGC00016480-07; NCGC00016480-08; NCGC00016480-09; NCGC00091036-01; NCGC00091036-02; NCGC00164322-01; NCGC00179275-01; NCGC00179275-02; NCGC00257464-01; NCGC00258717-01; BP-25390; HY-14590; KAEMPFEROL (CONSTITUENT OF GINKGO); Kaempferol 100 microg/mL in Acetonitrile; SY023424; AB00514046; FT-0614420; K0018; SW197199-2; 3,4',5,7-tetrahydroxy-Flavone (7CI,8CI); C05903; EN300-205764; H10428; S00111; Flavone, 3,4',5,7-tetrahydroxy- (7CI,8CI); KAEMPFEROL (CONSTITUENT OF GINKGO) [DSC]; A828886; Q393336; SR-01000765646; Kaempferol, primary pharmaceutical reference standard; Q-100584; SR-01000765646-3; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-chromen-4-one; BRD-K12807006-001-05-2; BRD-K12807006-001-10-2; Z57183373; 2-(4-hydroxyphenyl)-3,5,7-tris(oxidanyl)chromen-4-one; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one??; A91A6666-86C8-4B33-B3EF-F74CD3CD7F47; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1-benzopyran-4-one; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #; 4H-1-Benzopyran-4-one,5,7-trihydroxy-2-(4-hydroxyphenyl)-; Kaempferol, United States Pharmacopeia (USP) Reference Standard; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)- (9CI); 3,4',5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-5,7,4'-Trihydroxyflavonol
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
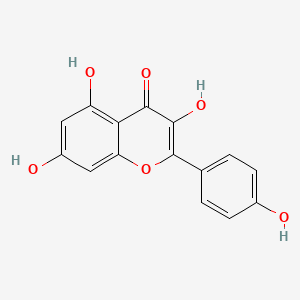 |
||||
| Formula |
C15H10O6
|
||||
| IUPAC Name |
3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
||||
| Canonical SMILES |
C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O
|
||||
| InChI |
InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
|
||||
| InChIKey |
IYRMWMYZSQPJKC-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cerebral ischaemic stroke | ICD-11: 8B11 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPCNs (Mouse primary cortical neurons) | ||||
| Response regulation | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male BALB/c mice (8-week-old, 20-22 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The experimental animals were fed adaptively for one week in the Experimental Animal Center of Guangdong Pharmaceutical University (Guangzhou, China). Feeding conditions were set at 26 , humidity 65% and a lightdark cycle for 12 hours. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Research Ethical Committee of Guangdong Pharmaceutical University (gdpulacspf2020007).
Click to Show/Hide
|
||||
| Response regulation | Kaempferol (KA) activated the Nrf2 pathway and upregulated Gpx4 in mouse livers and L02 cells to inhibit ferroptosis induced by APAP. Finally, molecular docking indicated the potential interaction of KA with Keap1. Taken together, KA ameliorated oxidative stress and ferroptosis-mediated acetaminophen-induced liver injury by activating Nrf2 signaling. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Cerebral ischaemic stroke | ICD-11: 8B11 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPCNs (Mouse primary cortical neurons) | ||||
| Response regulation | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male BALB/c mice (8-week-old, 20-22 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The experimental animals were fed adaptively for one week in the Experimental Animal Center of Guangdong Pharmaceutical University (Guangzhou, China). Feeding conditions were set at 26 , humidity 65% and a lightdark cycle for 12 hours. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Research Ethical Committee of Guangdong Pharmaceutical University (gdpulacspf2020007).
Click to Show/Hide
|
||||
| Response regulation | Kaempferol (KA) activated the Nrf2 pathway and upregulated Gpx4 in mouse livers and L02 cells to inhibit ferroptosis induced by APAP. Finally, molecular docking indicated the potential interaction of KA with Keap1. Taken together, KA ameliorated oxidative stress and ferroptosis-mediated acetaminophen-induced liver injury by activating Nrf2 signaling. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Cerebral ischaemic stroke | ICD-11: 8B11 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | mPCNs (Mouse primary cortical neurons) | |||
| Response regulation | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | |||
References
