Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0130)
| Name |
Propofol
|
||||
|---|---|---|---|---|---|
| Synonyms |
propofol; 2,6-DIISOPROPYLPHENOL; 2078-54-8; Diprivan; Disoprofol; Disoprivan; Fresofol; Diisopropylphenol; Ampofol; 2,6-Bis(1-methylethyl)phenol; Rapinovet; Propofolum; Ivofol; Recofol; Propofol-Lipuro; Phenol, 2,6-bis(1-methylethyl)-; Pofol; Diprifusor; Diprofol; Propovan; 2,6-di(propan-2-yl)phenol; ICI 35868; Phenol, 2,6-diisopropyl-; 2,6-Diisopropyl phenol; Diprivan 10; Propofolum [Latin]; Dipravan; Propoven; Aquafo; Diprivan Injectable emulsion; 2,6 Diisopropylphenol; NSC 5105; ICI-35868; 2,6-bis(propan-2-yl)phenol; CCRIS 9000; Lipuro; HSDB 7123; UNII-YI7VU623SF; EINECS 218-206-6; YI7VU623SF; ICI 35,868; BRN 1866484; 2,6-Di-iso-propylphenol-d18; DTXSID6023523; CHEBI:44915; AI3-26295; NSC5105; Phenol, 2,6-bis(1-methylethyl); 2,6-diisopropyl-phenol; ICI35,868; MFCD00008885; 2,6-dipropan-2-ylphenol; CHEMBL526; ICI 35-868; DTXCID103523; KETAFOL COMPONENT PROPOFOL; 4-06-00-03435 (Beilstein Handbook Reference); NSC-5105; Propofolum (Latin); NCGC00015389-08; Aquafol; CAS-2078-54-8; PROPOFOL (MART.); PROPOFOL [MART.]; PROPOFOL (USP-RS); PROPOFOL [USP-RS]; PFL; PROPOFOL (EP IMPURITY); PROPOFOL [EP IMPURITY]; PROPOFOL (EP MONOGRAPH); PROPOFOL (USP IMPURITY); PROPOFOL [EP MONOGRAPH]; PROPOFOL [USP IMPURITY]; PROPOFOL (USP MONOGRAPH); PROPOFOL [USP MONOGRAPH]; Propofol IDD-D; Diprivan (TN); SMR000059151; Propofol(2,6-Diisopropylphenol); 2, 6-Diisopropylphenol; SR-01000075468; 2,6-bis(Isopropyl)phenol; DDS-04F; ghl.PD_Mitscher_leg0.558; Propofol [USAN:USP:INN:BAN]; Propofol (Diprivan); ZD-0859; Phenol,6-diisopropyl-; PROPOFOL [HSDB]; PROPOFOL [USAN]; PROPOFOL [INN]; PROPOFOL [JAN]; PROPOFOL [MI]; PROPOFOL [VANDF]; Prestwick0_000931; Prestwick1_000931; Prestwick2_000931; Prestwick3_000931; 2,6-di isopropyl phenol; Phenol,2,6-diisopropyl-; Biomol-NT_000248; Lopac-D126608; PROPOFOL [WHO-DD]; Propofol (JAN/USP/INN); Lopac0_000437; SCHEMBL36245; BSPBio_000862; MLS001066348; MLS001335999; MLS002454360; BIDD:GT0436; PROPOFOL [GREEN BOOK]; SPECTRUM1505022; Propofol, 1mg/ml in Methanol; SPBio_003031; 2,6-Diisopropylphenol, 97%; PROPOFOL [ORANGE BOOK]; BPBio1_000950; BPBio1_000969; GTPL5464; Phenol,6-bis(1-methylethyl)-; Propofol, 10mg/ml in Methanol; Propofol, 50mg/ml in Methanol; N01AX10; Propofol 1.0 mg/ml in Methanol; Propofol, 100mg/ml in Methanol; 2,6-Diisopropylphenol, >=97%; 3f33; 3p50; HMS1570L04; HMS2089O21; HMS2094E17; HMS2097L04; HMS2231E16; HMS3259E03; HMS3261G16; HMS3369I16; HMS3714L04; Pharmakon1600-01505022; Phenol,2,6-bis(1-methylethyl)-; BCP02920; HY-B0649; Tox21_110134; Tox21_201371; Tox21_303225; Tox21_500437; AC8633; BDBM50058046; NSC758909; Phenol, 2, 6-bis(1-methylethyl)-; AKOS009159417; Tox21_110134_1; AC-2038; AM90311; CCG-204529; CS-W020057; DB00818; LP00437; NC00449; NSC-758909; SDCCGMLS-0318084.P029; SDCCGSBI-0050422.P002; 2,6-DIISOPROPYLPHENOL; PROPOFOL; MLS-0318084; NCGC00015389-01; NCGC00015389-02; NCGC00015389-03; NCGC00015389-04; NCGC00015389-05; NCGC00015389-06; NCGC00015389-07; NCGC00015389-09; NCGC00015389-10; NCGC00015389-11; NCGC00015389-14; NCGC00015389-17; NCGC00091538-01; NCGC00091538-02; NCGC00091538-03; NCGC00091538-04; NCGC00091538-05; NCGC00091538-06; NCGC00257228-01; NCGC00260670-01; NCGC00261122-01; AS-13299; SY013479; 2,6-Diisopropylphenol, analytical standard; BCP0726000298; MLS-0318084.P017; AB00513968; D0617; EU-0100437; EN300-52468; C07523; D00549; AB00513968-07; AB00513968_08; A814898; D126608; Q422740; Q-201631; SR-01000075468-1; SR-01000075468-4; SR-01000075468-6; BRD-K82255054-001-03-5; BRD-K82255054-001-08-4; Propofol, British Pharmacopoeia (BP) Reference Standard; Propofol, European Pharmacopoeia (EP) Reference Standard; Z752915492; Propofol, United States Pharmacopeia (USP) Reference Standard; Propofol, Pharmaceutical Secondary Standard; Certified Reference Material; InChI=1/C12H18O/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9,13H,1-4H; Propofol for peak identification, European Pharmacopoeia (EP) Reference Standard; 113981-41-2
Click to Show/Hide
|
||||
| Structure |
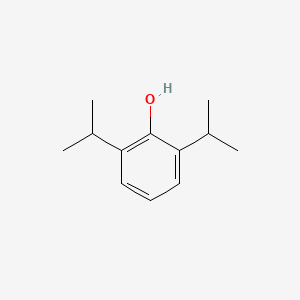 |
||||
| Formula |
C12H18O
|
||||
| IUPAC Name |
2,6-di(propan-2-yl)phenol
|
||||
| Canonical SMILES |
CC(C)C1=C(C(=CC=C1)C(C)C)O
|
||||
| InChI |
InChI=1S/C12H18O/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9,13H,1-4H3
|
||||
| InChIKey |
OLBCVFGFOZPWHH-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Regulator | hsa-miR-744-5p (miRNA) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (5 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no: SYXK (Beijing) 20170033). For tumor formation, 8 x 106 A549/Cis cells were subcutaneously injected into the right axilla of each mouse. On the 7th d, Cis (4.0 mg/kg) was intraperitoneally injected into each mouse every 4 days. Then, mice were allocated into 3 groups: Control group (no additional injection); SO group (intraperitoneal injection of soybean oil); and Propofol group [intraperitoneal injection of soybean oil-dissolved propofol (35 mg/kg)]. The volume of the tumor was measured by a caliper every 7 days. Tumor volume was measured according to the formula: V (mm3) = 1/2 ab2 (a: the longest axis of tumor; b: the shortest axis of tumor). Then 35 d after transplantation, mice were euthanatized to measure tumor weight using an electronic balance. A part of transplanted tumors was immediately conserved at liquid nitrogen and -80 . The rest was used for paraffin-embedding and immunohistochemical staining.
Click to Show/Hide
|
||||
| Response regulation | In summary, propofol inhibited GPX4-mediated ferroptosis and reduces CR of non-small cell lung cancer (NSCLC) cells to Cis through the miR-744-5p/miR-615-3p axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Regulator | hsa-miR-615-3p (miRNA) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (5 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no: SYXK (Beijing) 20170033). For tumor formation, 8 x 106 A549/Cis cells were subcutaneously injected into the right axilla of each mouse. On the 7th d, Cis (4.0 mg/kg) was intraperitoneally injected into each mouse every 4 days. Then, mice were allocated into 3 groups: Control group (no additional injection); SO group (intraperitoneal injection of soybean oil); and Propofol group [intraperitoneal injection of soybean oil-dissolved propofol (35 mg/kg)]. The volume of the tumor was measured by a caliper every 7 days. Tumor volume was measured according to the formula: V (mm3) = 1/2 ab2 (a: the longest axis of tumor; b: the shortest axis of tumor). Then 35 d after transplantation, mice were euthanatized to measure tumor weight using an electronic balance. A part of transplanted tumors was immediately conserved at liquid nitrogen and -80 . The rest was used for paraffin-embedding and immunohistochemical staining.
Click to Show/Hide
|
||||
| Response regulation | In summary, propofol inhibited GPX4-mediated ferroptosis and reduces CR of non-small cell lung cancer (NSCLC) cells to Cis through the miR-744-5p/miR-615-3p axis. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6 mice weighing 20-25 g each were obtained from the Animal Experimental Center of Yisi (Changchun, China). Mice were group-housed in a 12 h light/dark cycle (light between 08:00 and 20:00 h) in a temperature-controlled environment room (23-25 ). Mice had ad libitum access to food and water. All surgical procedures were carried out on animals anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. MCAO was achieved by inserting a silicone rubber-coated nylon monofilament into the internal carotid artery through the external carotid artery and temporary ligation of the right common carotid artery with a suture. After 45 min of ischemia, blood flow was restored by removing the filament and the suture, and the mice were allowed to recover for 24 h.
Click to Show/Hide
|
||||
| Response regulation | Our data support a protective role of propofol against ferroptosis as a cause of cell death in mice with cerebral ischemia-reperfusion injury. Propofol protected against cerebral ischemia-reperfusion injury-induced ferroptosis partly by regulating the Nrf2/Gpx4 signaling pathway. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response regulation | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6 mice weighing 20-25 g each were obtained from the Animal Experimental Center of Yisi (Changchun, China). Mice were group-housed in a 12 h light/dark cycle (light between 08:00 and 20:00 h) in a temperature-controlled environment room (23-25 ). Mice had ad libitum access to food and water. All surgical procedures were carried out on animals anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. MCAO was achieved by inserting a silicone rubber-coated nylon monofilament into the internal carotid artery through the external carotid artery and temporary ligation of the right common carotid artery with a suture. After 45 min of ischemia, blood flow was restored by removing the filament and the suture, and the mice were allowed to recover for 24 h.
Click to Show/Hide
|
||||
| Response regulation | Our data support a protective role of propofol against ferroptosis as a cause of cell death in mice with cerebral ischemia-reperfusion injury. Propofol protected against cerebral ischemia-reperfusion injury-induced ferroptosis partly by regulating the Nrf2/Gpx4 signaling pathway. | ||||
NAD(P)H dehydrogenase [quinone] 1 (NQO1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response regulation | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response regulation | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response regulation | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
References
