Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0341)
| Name |
GW4869
|
||||
|---|---|---|---|---|---|
| Synonyms |
GW4869; 6823-69-4; GW69A; GW 4869; GW-4869 HCl; GW4869 dihydrochloride; 6823-69-4 (HCl); GW-4869; (2E,2'E)-3,3'-(1,4-phenylene)bis(N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenyl)acrylamide) dihydrochloride; p-Benzenediacrylanilide, 4 inverted exclamation marka,4 inverted exclamation marka inverted exclamation marka-di-2-imidazolin-2-yl-, dihydrochloride; GW554869A; N-SMase Inhibitor; (E)-3-[4-[(E)-3-[4-(4,5-dihydro-1H-imidazol-2-yl)anilino]-3-oxoprop-1-enyl]phenyl]-N-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]prop-2-enamide; EX-A1154; MFCD06411564; CS-6865; BS-17430; HY-19363; SR-01000946355; SR-01000946355-1; (E)-3-[4-[(E)-3-[4-(4,5-dihydro-1H-imidazol-2-yl)anilino]-3-oxoprop-1-enyl]phenyl]-N-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]prop-2-enamide;dihydrochloride; 2-Propenamide, 3,3'-(1,4-phenylene)bis[N-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-, dihydrochloride; p-Benzenediacrylanilide,4invertedexclamationmarka,4invertedexclamationmarkainvertedexclamationmarka-di-2-imidazolin-2-yl-,dihydrochloride
Click to Show/Hide
|
||||
| Structure |
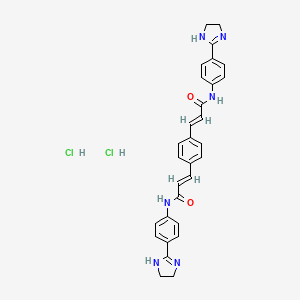 |
||||
|
3D MOL
|
|||||
| Formula |
C30H30Cl2N6O2
|
||||
| IUPAC Name |
(E)-3-[4-[(E)-3-[4-(4,5-dihydro-1H-imidazol-2-yl)anilino]-3-oxoprop-1-enyl]phenyl]-N-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]prop-2-enamide;dihydrochloride
|
||||
| Canonical SMILES |
C1CN=C(N1)C2=CC=C(C=C2)NC(=O)C=CC3=CC=C(C=C3)C=CC(=O)NC4=CC=C(C=C4)C5=NCCN5.Cl.Cl
|
||||
| InChI |
InChI=1S/C30H28N6O2.2ClH/c37-27(35-25-11-7-23(8-12-25)29-31-17-18-32-29)15-5-21-1-2-22(4-3-21)6-16-28(38)36-26-13-9-24(10-14-26)30-33-19-20-34-30;;/h1-16H,17-20H2,(H,31,32)(H,33,34)(H,35,37)(H,36,38);2*1H/b15-5+,16-6+;;
|
||||
| InChIKey |
NSFKAZDTKIKLKT-CLEIDKRQSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia | ICD-11: BC81 | |||
| Responsed Regulator | rno-miR-23a-3p (miRNA) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| rCFs (Rat cardiac fibroblasts) | |||||
| In Vivo Model |
Eighteen beagles, randomly divided into three groups, both sexes and an average age of 1 year, weighing 7.5 ± 1.5 kg, were used for the study as follows: Sham group (n = 6), Pacing group (n = 6), and GW4869 + Pacing group (n = 6). Each beagle canine was given an intramuscular injection of 25 mg/kg ketamine sulfate before being premedicated with pentobarbital sodium (30 mg/kg, intravenous injection) and ventilated with room air by a respirator (MAO01746, Harvard Apparatus Holliston, United States). Venous access was established to supply saline (50-100 mL/h) or pentobarbital sodium (2.5 mg/kg/h).
Click to Show/Hide
|
||||
| Response regulation | The exosome inhibitor GW4869 reduced ferroptosis, fibrosis, and inflammation and improved histological and electrophysiological remodeling. Pacing-CF-exos highly expressed miR-23a-3p by informatics analysis and experimental verification. Inhibitor- miR-23a-3p protected h9c2 cells from ferroptosis accompanying with upregulation of SLC7A11. The development of atrial fibrillation (AF) in a persistent direction could be prevented by intervening with exosomal miRNAs to reduce oxidative stress injury and ferroptosis. | ||||
