Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0163)
| Name |
Formononetin
|
||||
|---|---|---|---|---|---|
| Synonyms |
formononetin; 485-72-3; Biochanin B; Formononetol; 7-hydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one; 7-Hydroxy-4'-methoxyisoflavone; 7-hydroxy-3-(4-methoxyphenyl)chromen-4-one; Flavosil; Neochanin; Myconate; Mycotech; 4'-O-methyldaidzein; 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-methoxyphenyl)-; Isoflavone, 7-hydroxy-4'-methoxy-; NSC 93360; NSC-93360; 7-Hydroxy-3-(4-methoxyphenyl)-4-benzopyrone; CHEBI:18088; EINECS 207-623-9; UNII-295DQC67BJ; MFCD00016948; 295DQC67BJ; DTXSID4022311; 7-hydroxy-4'-methoxy-isoflavone; 7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one; DTXCID502311; NSC93360; 7-hydroxy-3-(4-methoxyphenyl)-4H-benzopyran-4-one; FORMONONETIN (USP-RS); FORMONONETIN [USP-RS]; SMR000470932; 7-Hydroxy-3-(4-methoxyphenyl)chromone; SR-01000765510; formononetine; Formoononetin; Formononetin,(S); Spectrum_000373; SpecPlus_000223; Daidzein 4-methyl ether; Spectrum2_000560; Spectrum3_000660; Spectrum4_001429; Spectrum5_000258; FORMONONETIN [MI]; Formononetin (Formononetol); FORMONONETIN [INCI]; NCIOpen2_005983; Oprea1_139748; Oprea1_815287; SCHEMBL62915; BSPBio_002299; KBioGR_001878; KBioSS_000853; SPECTRUM102007; MLS000697593; MLS006011897; BIDD:ER0119; DivK1c_006319; SPBio_000639; CHEMBL242341; Formononetin, analytical standard; KBio1_001263; KBio2_000853; KBio2_003421; KBio2_005989; KBio3_001519; HMS1922N18; HMS2231I04; HMS3369C07; HMS3655N22; BCP29929; Formononetin, >=99.0% (TLC); HY-N0183; TNP00176; Tox21_301848; BBL010458; BDBM50021398; CCG-38727; LMPK12050037; s2299; STK801612; AKOS000270811; AC-8001; DB15335; SDCCGMLS-0066428.P001; 7-hydroxy-4'-methoxy-Isoflavone (8CI); NCGC00017269-01; NCGC00017269-02; NCGC00017269-03; NCGC00017269-04; NCGC00017269-05; NCGC00017269-06; NCGC00017269-07; NCGC00095207-01; NCGC00095207-02; NCGC00095207-03; NCGC00178715-01; NCGC00255167-01; AS-11642; CAS-485-72-3; NCI60_042081; F0868; FT-0626540; FT-0632204; K-080; SW219915-1; C00858; EN300-116214; FORMONONETIN (CONSTITUENT OF ASTRAGALUS); AB00052676-07; FORMONONETIN (CONSTITUENT OF RED CLOVER); A827555; AE-641/01968055; Q408859; 7-hydroxy-3-(4-methoxyphenyl)-1-benzopyran-4-one; Q-100540; SR-01000765510-3; SR-01000765510-4; 7-Hydroxy-3-(4'-methoxyphenyl)-4H-benzopyran-4-one; BRD-K55567017-001-02-4; BRD-K55567017-001-06-5; FORMONONETIN (CONSTITUENT OF ASTRAGALUS) [DSC]; FORMONONETIN (CONSTITUENT OF RED CLOVER) [DSC]; F3139-1207; Z374511822; 7-hydroxy-3-(4-methoxyphenyl)-4H-benzopyran-4-one(9CI); Formononetin, United States Pharmacopeia (USP) Reference Standard; 7-Hydroxy-3-(4-methoxyphenyl)chromone, 7-Hydroxy-4'-methoxyisoflavone; Biochanin B; Flavosil; Formononetol; NSC 93360; NSC93360; NSC-93360
Click to Show/Hide
|
||||
| Structure |
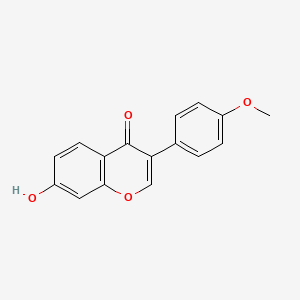 |
||||
| Formula |
C16H12O4
|
||||
| IUPAC Name |
7-hydroxy-3-(4-methoxyphenyl)chromen-4-one
|
||||
| Canonical SMILES |
COC1=CC=C(C=C1)C2=COC3=C(C2=O)C=CC(=C3)O
|
||||
| InChI |
InChI=1S/C16H12O4/c1-19-12-5-2-10(3-6-12)14-9-20-15-8-11(17)4-7-13(15)16(14)18/h2-9,17H,1H3
|
||||
| InChIKey |
HKQYGTCOTHHOMP-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Chronic kidney disease | ICD-11: GB61 | |||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response regulation | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/ Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Chronic kidney disease | ICD-11: GB61 | |||
| Responsed Regulator | Cyclic AMP-dependent transcription factor ATF-3 (ATF3) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response regulation | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
