Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0206)
| Name |
Salidroside
|
||||
|---|---|---|---|---|---|
| Synonyms |
Salidroside; 10338-51-9; Rhodioloside; Rhodosin; sallidroside; salidroside, (-)-; Glucopyranoside, p-hydroxyphenethyl; Tyrosol glucoside; (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]oxane-3,4,5-triol; (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-(4-hydroxyphenethoxy)tetrahydro-2H-pyran-3,4,5-triol; UNII-M983H6N1S9; 2-(4-Hydroxyphenyl)ethyl beta-D-glucopyranoside; DTXSID4049034; M983H6N1S9; NSC-741643; 8-O-b-D-glucoside of tyrosol; CHEBI:9009; CHEMBL465208; DTXCID9028960; beta-D-Glucopyranoside, 2-(4-hydroxyphenyl)ethyl; NSC 741643; MFCD00210553; p-Hydroxyphenethyl alcohol 1-O-beta-D-glucoside; SALIDROSIDE (USP-RS); SALIDROSIDE [USP-RS]; CAS-10338-51-9; p-Hydroxyphenethyl glucopyranoside; Salidroside,(S); NCGC00169145-02; pyran-3,4,5-triol; 2-(4-hydroxyphenyl)ethyl-beta-d-glucopyranoside; salidroside (rhodioloside); Salidroside - Rhodioloside; beta-D-glucopyranoside, 2-(4-hydroxyphenyl)ethyl-; MLS006010734; SCHEMBL148079; MEGxp0_000478; Salidroside, analytical standard; ACon1_000366; 2-(hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]tetrahydropyran-3,4,5-triol; HMS3884N15; AMY22501; HY-N0109; Tox21_113565; (4-hydroxyphenethoxy)tetrahydro-2H-; BDBM50269651; NSC741643; s2396; 4-hydroxyphenyl-2-ethylglucopyranoside; AKOS015895134; Tox21_113565_1; AC-6071; CCG-267467; CS-5300; Salidroside, >=95% (LC/MS-ELSD); NCGC00169145-01; NCGC00169145-03; SMR001294679; TYROSOL alpha-(beta-D-GLUCOPYRANOSIDE); SW219124-1; 2-(4-Hydroxyphenyl)ethyl ?-D-glucopyranoside; (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-; C06046; 2-(4-Hydroxyphenyl)ethyl bet.-D-glucopyranoside; A800725; SALIDROSIDE (CONSTITUENT OF RHODIOLA ROSEA); Q-100031; Q7404463; TYROSOL .ALPHA.-(.BETA.-D-GLUCOPYRANOSIDE); BRD-K66030860-001-01-0; Salidroside, primary pharmaceutical reference standard; 2-(4-HYDROXYPHENYL)ETHYL-.BETA.-D-GLUCOPYRANOSIDE; SALIDROSIDE (CONSTITUENT OF RHODIOLA ROSEA) [DSC]; .BETA.-D-GLUCOPYRANOSIDE, 2-(4-HYDROXYPHENYL)ETHYL-; Salidroside, United States Pharmacopeia (USP) Reference Standard; (2R,3R,4S,5S,6R)-2-[2-(4-hydroxyphenyl)ethoxy]-6-methylol-tetrahydropyran-3,4,5-triol; (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]tetrahydropyran-3,4,5-triol
Click to Show/Hide
|
||||
| Structure |
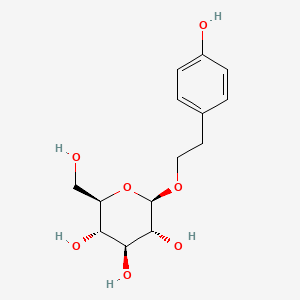 |
||||
| Formula |
C14H20O7
|
||||
| IUPAC Name |
(2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]oxane-3,4,5-triol
|
||||
| Canonical SMILES |
C1=CC(=CC=C1CCOC2C(C(C(C(O2)CO)O)O)O)O
|
||||
| InChI |
InChI=1S/C14H20O7/c15-7-10-11(17)12(18)13(19)14(21-10)20-6-5-8-1-3-9(16)4-2-8/h1-4,10-19H,5-7H2/t10-,11-,12+,13-,14-/m1/s1
|
||||
| InChIKey |
ILRCGYURZSFMEG-RKQHYHRCSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung injury | ICD-11: NB32 | |||
| Responsed Regulator | Mitogen-activated protein kinase 1 (MAPK1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mLT (Mouse lung tissue) | ||||
| In Vivo Model |
In our study, the 32 mice were randomly divided for four groups (n = 8 per group): (1) room-air-expose (sham), (2) hyperoxia-expose with Sal (Sal + Hyperoxia), (3) hyperoxia-exposed (Hyperoxia), (4) hyperoxia-exposed with Y-320 (an inhibitor of IL-17) (Y-320 + Hyperoxia). The mice exposed to normoxia groups were placed in room air with 21% oxygen, and the mice exposed to hyperoxia were placed in over 90% oxygen for 24 h. The continue exposure to over 90% oxygen was achieved in a self-made airtight box which attached to a medical oxygen cylinder, and the O2 level inside was continuously monitored with O2 analyzer, mice had free access to food and water. In the first three days before exposure to the hyperoxia, mice in the Sal + Hyperoxia group or Y-320 + Hyperoxia group were treated with Sal (100 mg/Kg) or Y-320 (2 mg/Kg) once orally every day, while the rest of groups were given equal isotonic saline. Based on the above experiments, eight 8-week-old KM mice were randomly divided into two groups: Sal + Hyperoxia group and Sal + Hyperoxia + IL-17A group. Sal + Hyperoxia + IL-17A group, mice were i.v. injected with 50 ug/kg of recombinant mouse IL-17A (210-17, Pepro Tech, USA). Animal were sacrificed following reperfusion, and lungs were stored at -80 until further experimental analysis.
Click to Show/Hide
|
||||
| Response regulation | When we applied recombinant IL-17A in Sal+hyperoxia group mice, the protein levels of IL-17RA, Act1, TRAF6, p38 MAPK and p-p38 MAPK increased significantly, and the expression level of GPX4 significantly decreased. Therefore, we demonstrated that IL-17A/IL-17RA mediates ferroptosis of AECII, least in part, via Act1/TRAF6/p38 MAPK pathway, which is responsible for the protective effects of salidroside on hyperoxia-induced acute lung injury (HALI). | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Diabetes mellitus | ICD-11: 5A10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | |||||
| MOVAS-1 cells | Normal | Homo sapiens | CVCL_0F08 | ||
| In Vivo Model |
For diabetes induction, C57BL/6 mice were given a high-fat diet for three weeks that contained 20% protein, 20% carbohydrate, and 60% fat. Sodium citratebuffer-diluted 60 mg/kg body weight streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) were administered intraperitoneally for the next constitutive six days. Prior to each STZ injection and blood glucose testusing Accu-Check Integra (Roche Diagnostics, Shanghai, China), mice were fasted overnight. Mice with blood glucose levels higher than 16.6 mM were considered diabetic and were utilized to establish the diabetic HLI model.
Click to Show/Hide
|
||||
| Response regulation | Salidroside/GPX4-mediated ferroptosis inhibition is crucial for promoting angiogenesis and blood perfusion recovery in diabetic hindlimb ischemia mice. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CD8T cells (Mouse CD8+ T cells) | ||||
| In Vivo Model |
SAMP8 mice were employed as an AD model and were treated with salidroside for 12 weeks. Behavioral tests, immunohistochemistry, HE and Nissl staining, immunofluorescence, transmission electron microscopy, quantitative proteomics, bioinformatic analysis, flow cytometry, iron staining,western blotting, andmolecular dockingwere performed.
Click to Show/Hide
|
||||
| Response regulation | Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis in Alzheimer's disease. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8T cells infiltration. | ||||
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | 5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57/BL mice (aged between 6 and 8 weeks and weighing 20 ± 2 g) were randomly divided into 6 groups (n = 6 mice per group) with equal number of mice in each group. The groups included saline control group (control group): 200 ulxd-1 saline intraperitoneally administered to the mice for 10 days; DOX model group (DOX group): 200 ulxd-1 saline intraperitoneally administered to the mice for 10 days and a single intraperitoneal administration of 10 mgxkg-1 DOX (HY-15,142, MCE, China) to the mice on the seventh day.
Click to Show/Hide
|
||||
| Response regulation | Salidroside markedly down-regulated ferroptotic cell death by activating AMPK-dependent signaling pathways including regulating abnormal fatty acid metabolism and maintaining mitochondrial function. Therefore, salidroside is can be exploited to develop a novel medication for clinical Doxorubicin-induced cardiotoxicity. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CD8T cells (Mouse CD8+ T cells) | ||||
| In Vivo Model |
SAMP8 mice were employed as an AD model and were treated with salidroside for 12 weeks. Behavioral tests, immunohistochemistry, HE and Nissl staining, immunofluorescence, transmission electron microscopy, quantitative proteomics, bioinformatic analysis, flow cytometry, iron staining,western blotting, andmolecular dockingwere performed.
Click to Show/Hide
|
||||
| Response regulation | Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis in Alzheimer's disease. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8T cells infiltration. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
B6.1291-Nfe2l2tm1Ywk/J (Nrf2-/-mice, 017009)and wild-type C57BL/6 were originally from the Jackson Laboratory. Grouping and administration were started when weighing approximately 28-33 g. All mice were housed in a laboratory environment with free access to adequate food and water under a 12 h/12 h light/dark cycle at 22 ± 1 and 55 ± 5% humidity. All procedures conformed to the protocols of the Animal Welfare Commission and Ethical Committee of Southern Medical University. The Nrf2-/- mice were identified by genotyping as shown in Fig.1B. WT and Nrf2-/- mice were randomly assigned to 32 groups (3 groups for WT mice and 3 groups for Nrf2-/- mice) as follows: a sham group, sham + Ab1-42 group, and Salidroside + Ab1-42 group.
Click to Show/Hide
|
||||
| Response regulation | Salidroside plays a neuroprotective role by inhibiting neuronal ferroptosis in A1-42-induced Alzheimer's disease mice and Glu-injured HT22 cells, and its mechanism is related to activation of the Nrf2/HO1 signaling pathway. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Following endotracheal intubation, mice were ventilated with room air at a rate of 120 cycles/min and atidal volumeof 7 mL/kg (MiniVent, Harvard Apparatus, USA). To induce ischemia, mice underwent left thoracotomy, and the left pulmonary hilum was blocked for 60 min with a microvascular clamp. After ischemia, the coronary artery was reperfused for 120 min by removing the clamp. The mice were euthanized at the end of the experiment through CO2 asphyxiation and cervical dislocation. Next, bronchoalveolar lavage fluid (BALF), blood, and lung samples were collected for testing.
Click to Show/Hide
|
||||
| Response regulation | Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
B6.1291-Nfe2l2tm1Ywk/J (Nrf2-/-mice, 017009)and wild-type C57BL/6 were originally from the Jackson Laboratory. Grouping and administration were started when weighing approximately 28-33 g. All mice were housed in a laboratory environment with free access to adequate food and water under a 12 h/12 h light/dark cycle at 22 ± 1 and 55 ± 5% humidity. All procedures conformed to the protocols of the Animal Welfare Commission and Ethical Committee of Southern Medical University. The Nrf2-/- mice were identified by genotyping as shown in Fig.1B. WT and Nrf2-/- mice were randomly assigned to 32 groups (3 groups for WT mice and 3 groups for Nrf2-/- mice) as follows: a sham group, sham + Ab1-42 group, and Salidroside + Ab1-42 group.
Click to Show/Hide
|
||||
| Response regulation | Salidroside plays a neuroprotective role by inhibiting neuronal ferroptosis in A1-42-induced Alzheimer's disease mice and Glu-injured HT22 cells, and its mechanism is related to activation of the Nrf2/HO1 signaling pathway. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Following endotracheal intubation, mice were ventilated with room air at a rate of 120 cycles/min and atidal volumeof 7 mL/kg (MiniVent, Harvard Apparatus, USA). To induce ischemia, mice underwent left thoracotomy, and the left pulmonary hilum was blocked for 60 min with a microvascular clamp. After ischemia, the coronary artery was reperfused for 120 min by removing the clamp. The mice were euthanized at the end of the experiment through CO2 asphyxiation and cervical dislocation. Next, bronchoalveolar lavage fluid (BALF), blood, and lung samples were collected for testing.
Click to Show/Hide
|
||||
| Response regulation | Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. | ||||
References
