Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0335)
| Name |
RSL3
|
||||
|---|---|---|---|---|---|
| Synonyms |
RSL3; 1219810-16-8; 1S,3R-RSL3; RSL3 1S,3R-; (1S,3R)-RSL3; RSL3 (1S,3R-); CHEMBL4747331; (1S,3R)-Methyl 2-(2-chloroacetyl)-1-(4-(methoxycarbonyl)phenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate; methyl (1S,3R)-2-(2-chloroacetyl)-1-(4-(methoxycarbonyl)phenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate; methyl (1S,3R)-2-(2-chloroacetyl)-1-[4-(methoxycarbonyl)phenyl]-1H,2H,3H,4H,9H-pyrido[3,4-b]indole-3-carboxylate; methyl (1S,3R)-2-(2-chloroacetyl)-1-(4-methoxycarbonylphenyl)-1,3,4,9-tetrahydropyrido[3,4-b]indole-3-carboxylate; RSL3 1S; SCHEMBL13402744; EX-A1392; BDBM50547192; NSC833292; s8155; AKOS030632788; CCG-269132; CS-5650; HY-100218A; NSC-833292; CID 1750826; AC-35345; AS-55795; K601; C21479; C71823; EN300-7365470; Q55879537; (1S,3R)-Methyl 2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1-[4-(methoxycarbonyl)phenyl]-1H-pyrido[3,4-b]indole-3-carboxylate
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
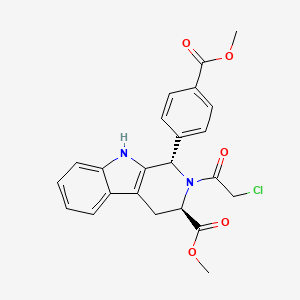 |
||||
| Formula |
C23H21ClN2O5
|
||||
| IUPAC Name |
methyl (1S,3R)-2-(2-chloroacetyl)-1-(4-methoxycarbonylphenyl)-1,3,4,9-tetrahydropyrido[3,4-b]indole-3-carboxylate
|
||||
| Canonical SMILES |
COC(=O)C1CC2=C(C(N1C(=O)CCl)C3=CC=C(C=C3)C(=O)OC)NC4=CC=CC=C24
|
||||
| InChI |
InChI=1S/C23H21ClN2O5/c1-30-22(28)14-9-7-13(8-10-14)21-20-16(15-5-3-4-6-17(15)25-20)11-18(23(29)31-2)26(21)19(27)12-24/h3-10,18,21,25H,11-12H2,1-2H3/t18-,21+/m1/s1
|
||||
| InChIKey |
TXJZRSRTYPUYRW-NQIIRXRSSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | |||
| Responsed Regulator | Nonsense-mediated mRNA decay factor SMG9 (SMG9) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-oldathymic nude mice(n = 5 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intratumoral treatment with RSL3 (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | SMG9, a component of the NMD machinery, is a selective driver for ferroptosis in pancreatic cancer cells. SMG9 is a direct binding protein of GPX4 to promote the degradation of GPX4 in response to RSL3 (a GPX4 inhibitor), but not erastin (a SLC7A11 inhibitor). | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response regulation | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| Response regulation | RSL3 triggered ferroptotic cell death by promoting the accumulation of cellular ROS and increasing the cellular LIP level. Mechanismly, we found transferrin expression were elevated in colorectal cancer cells treated with RSL3 accompanied by a decrease in the expression of GPX4, indicating an iron-dependent cell death. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Regulator | Ubiquitin carboxyl-terminal hydrolase 11 (USP11) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H2122 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1531 | ||
| In Vivo Model |
After two weeks in house, the mice were subcutaneously injected with A549 cells (100 uL containing 5 x 106 cells/injection) and monitored for tumor cell xenografts to reach approximately 100 mm3. The mice were then divided into two groups (n = 5), the RSL3 treatment (100 mg/kg; dissolved in 5% dimethyl sulfoxide/corn oil; administrated intratumorally twice a day for one week) and control (5% dimethyl sulfoxide/corn oil only) groups.
Click to Show/Hide
|
||||
| Response regulation | RSL3 was able to directly bind to USP11, a recently identified de-ubiquitinase of NRF2, and inactivate USP11 protein to induce NRF2 protein ubiquitination and degradation in KLK lung adenocarcinoma cells. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | NF-kappa-B inhibitor alpha (NFKBIA) | Driver | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response regulation | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Cyclic AMP-dependent transcription factor ATF-4 (ATF4) | Suppressor | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response regulation | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | |||
| Responsed Regulator | Ceruloplasmin (CP) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| Response regulation | Erastin and RSL3 suppress ceruloplasmin expression in hepatocellular carcinoma cells. CP suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. The suppression function of ceruloplasmin in erastin- and RSL3-induced ferroptosis is dependent on FPN. | ||||
References
