Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0275)
| Name |
Manoalide
|
||||
|---|---|---|---|---|---|
| Synonyms |
manoalide; 75088-80-1; CHEMBL463914; CHEBI:66666; E1DK0157K9; (2R)-2-hydroxy-3-[(2R,6R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethylcyclohexen-1-yl)hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl]-2H-furan-5-one; UNII-E1DK0157K9; SCHEMBL20551728; DTXSID401028174; HY-N7487; BDBM50250399; AT25841; 2(5H)-Furanone, 4-((2R,6R)-3,6-dihydro-6-hydroxy-5-((3E)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-hexenyl)-2H-pyran-2-yl)-5-hydroxy-, (5R)-; 2(5H)-Furanone, 4-(3,6-dihydro-6-hydroxy-5-(4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-hexenyl)-2H-pyran-2-yl)-5-hydroxy-; CS-0129960; C17156; HMP Linker?4-(Hydroxymethyl) Phenoxyacetic Acid; A838321; Q10853851; (2R)-2-hydroxy-3-[(2R,6R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexenyl)hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl]-2H-furan-5-one; (2R)-3-[(2R,6R)-5-[(E)-4-methyl-6-(2,6,6-trimethylcyclohexen-1-yl)hex-3-enyl]-6-oxidanyl-3,6-dihydro-2H-pyran-2-yl]-2-oxidanyl-2H-furan-5-one; (5R)-5-HYDROXY-4-((2R,6R)-6-HYDROXY-5-((E)-4-METHYL-6-(2,6,6-TRIMETHYL-1-CYCLOHEXENYL)HEX-3-ENYL)-3,6-DIHYDRO-2H-PYRAN-2-YL)-5H-FURAN-2-ONE; (5R)-5-hydroxy-4-{(2R,6R)-6-hydroxy-5-[(3E)-4-methyl-6-(2,6,6-trimethylcyclohex-1-en-1-yl)hex-3-en-1-yl]-3,6-dihydro-2H-pyran-2-yl}furan-2(5H)-one; 2(5H)-Furanone, 4-[(2R,6R)-3,6-dihydro-6-hydroxy-5-[(3E)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-hexen-1-yl]-2H-pyran-2-yl]-5-hydroxy-, (5R)-; 5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one
Click to Show/Hide
|
||||
| Status |
Phase 2
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
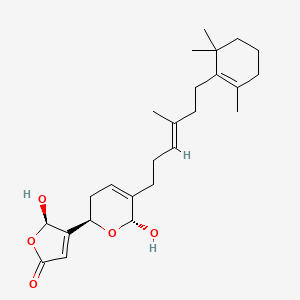 |
||||
| Formula |
C25H36O5
|
||||
| IUPAC Name |
(2R)-2-hydroxy-3-[(2R,6R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethylcyclohexen-1-yl)hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl]-2H-furan-5-one
|
||||
| Canonical SMILES |
CC1=C(C(CCC1)(C)C)CCC(=CCCC2=CCC(OC2O)C3=CC(=O)OC3O)C
|
||||
| InChI |
InChI=1S/C25H36O5/c1-16(10-12-20-17(2)8-6-14-25(20,3)4)7-5-9-18-11-13-21(29-23(18)27)19-15-22(26)30-24(19)28/h7,11,15,21,23-24,27-28H,5-6,8-10,12-14H2,1-4H3/b16-7+/t21-,23-,24-/m1/s1
|
||||
| InChIKey |
FGJIDQWRRLDGDB-CPIXEKRISA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
The LSL-KrasG12D mouse model was obtained from the Jackson Laboratory (Sacramento, CA). Adeno-Cre (Genechem, Shanghai, China) was introduced into the trachea of mice at a dose of 1.25 x 1011 PFU in a total volume of 50 uL. Tumor tissues from 12-week post-infection mice were washed with cold PBS, cut into small pieces, and washed with DMEM/F12 (containing 1 x Glutamine, 10 mM HEPES, and antibiotics), digested with collagenase I and IV for 0.5-1 h at 37. After washing twice with DMEM/F12 and centrifugation (500 g, 5 min), the dissociated cells were seeded into growth factor-reduced matrigel (Corning, #356237) at 37 for 30 min.
Click to Show/Hide
|
||||
| Response regulation | Manoalide (MA) induces ferroptosis by suppressing the NRF2-SLC7A11 axis and mitochondrial Ca2+overload induced-FTH1 pathways to promote the sensitivity of osimertinib-resistant lung cancer cells to osimertinib. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
The LSL-KrasG12D mouse model was obtained from the Jackson Laboratory (Sacramento, CA). Adeno-Cre (Genechem, Shanghai, China) was introduced into the trachea of mice at a dose of 1.25 x 1011 PFU in a total volume of 50 uL. Tumor tissues from 12-week post-infection mice were washed with cold PBS, cut into small pieces, and washed with DMEM/F12 (containing 1 x Glutamine, 10 mM HEPES, and antibiotics), digested with collagenase I and IV for 0.5-1 h at 37. After washing twice with DMEM/F12 and centrifugation (500 g, 5 min), the dissociated cells were seeded into growth factor-reduced matrigel (Corning, #356237) at 37 for 30 min.
Click to Show/Hide
|
||||
| Response regulation | Manoalide (MA) induces ferroptosis by suppressing the NRF2-SLC7A11 axis and mitochondrial Ca2+overload induced-FTH1 pathways to promote the sensitivity of osimertinib-resistant lung cancer cells to osimertinib. | ||||
