Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0051)
| Name |
Doxorubicin
|
||||
|---|---|---|---|---|---|
| Synonyms |
doxorubicin; Adriamycin; 23214-92-8; Adriablastin; Doxil; Doxorubicine; Doxorubicinum; Adriblastina; 14-Hydroxydaunomycin; Hydroxydaunorubicin; Doxorubicina; 14-Hydroxydaunorubicine; Adriamycin semiquinone; Doxorubicine [INN-French]; Doxorubicinum [INN-Latin]; Doxorubicina [INN-Spanish]; Caelyx; Doxorubicin HCl; CCRIS 739; Adriblastin; HSDB 3070; NCI-C01514; NDC 38242-874; EINECS 245-495-6; FI 106; Resmycin; CHEBI:28748; UNII-80168379AG; NSC-759155; CHEMBL53463; Caelyx (liposomal doxorubicin); (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; 80168379AG; DTXSID8021480; (1S,3S)-3-Glycoloyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl-(3-amino-2,3,6-tridesoxy-alpha-L-lyxo-hexopyranosid); (1S,3S)-3-glycoloyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (8S,10S)-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione; (8S,10S)-10-((3-Amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione; 1,2,3,4,6,11-Hexahydro-4beta,5,12-trihydroxy-4-(hydroxyacetyl)-10-methoxy-6,11-dioxonaphthacen-1beta-yl-3-amino-2,3,6-trideoxy-alpha-L-lyxohexopyranoside; 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-; DM2; ADM; NSC-123127; DOXORUBICIN (MART.); DOXORUBICIN [MART.]; (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione; (8S-cis)-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione; Doxorubicin [USAN:INN:BAN]; ThermoDox; hydroxydaunomycin; MLS000028393; Doxorubicin-hLL1; RDF Rubex; (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)-; Adriblastina (TN); Doxorubicin-P4/D10; Doxorubicin (USAN/INN); VALRUBICIN IMPURITY, DOXORUBICIN (USP IMPURITY); VALRUBICIN IMPURITY, DOXORUBICIN [USP IMPURITY]; Doxorubicin-hLL1 conjugate; doxorrubicina; Doxorubicin-P4/D10 conjugate; ADR; Hydroxyldaunorubicin; Hydroxyl Daunorubicin; NSC123127; DOXORUBICIN [MI]; (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; Prestwick0_000438; Prestwick1_000438; Prestwick2_000438; Prestwick3_000438; DOXORUBICIN [INN]; DOXORUBICIN [HSDB]; DOXORUBICIN [USAN]; Probes1_000151; Probes2_000129; DOXORUBICIN [VANDF]; SCHEMBL3243; BSPBio_000456; BSPBio_001031; DOXORUBICIN [WHO-DD]; 10-((3-Amino-2,3,6-trideoxy-D-lyxohexopyranosyl)oxy)-8-glycolcyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione; SPBio_002395; (8S-cis)-10-; BPBio1_000502; cid_443939; DTXCID301480; GTPL7069; Valrubicin impurity, doxorubicin; BDBM22984; BDBM32022; L01DB01; AOJJSUZBOXZQNB-TZSSRYMLSA-N; HMS2089H06; (8S,10S)-10-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-8-GLYCOLOYL-7,8,9,10-TETRAHYDRO-6,8,11-TRIHYDROXY-1-METHOXY-5,12-NAPHTHACENEDIONE; 5,12-NAPHTHACENEDIONE, 10-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-7,8,9,10-TETRAHYDRO-6,8,11-TRIHYDROXY-8-(HYDROXYACETYL)-1-METHOXY-, (8S-CIS)-; GR-319; HY-15142A; LMPK13050001; AKOS015951330; Conjugate of doxorubicin with humanized monoclonal antibody LL1 against CD74; Conjugate of doxorubicin with monoclonal antibody P4/D10 against GP120; DB00997; SMP1_000106; NCGC00024415-35; NCGC00024415-37; NCGC00024415-38; NCGC00024415-40; NCGC00024415-41; NCGC00024415-42; NCGC00024415-61; BP-23114; (8S,10S)-10; (8S,10S)-10-; A14403; C01661; D03899; EN300-120698; Epirubicin hydrochloride impurity, doxorubicin-; H11954; Q18936; A816625; BRD-K92093830-003-04-3; BRD-K92093830-003-25-8; EPIRUBICIN HYDROCHLORIDE IMPURITY C [EP IMPURITY]; DAUNORUBICIN HYDROCHLORIDE IMPURITY D [EP IMPURITY]; EPIRUBICIN HYDROCHLORIDE IMPURITY, DOXORUBICIN- [USP IMPURITY]; (7S,9R)-7-[(2S,4S,5S,6S)-4-Amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-9-glycoloyl-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride; (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-4-methoxy-6,9,11-tris(oxidanyl)-9-(2-oxidanylethanoyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; (7S,9S)-7-[(4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-9-(2-hydroxy-1-oxoethyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; (8S,10S)-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione;(7S,9S)-7-[(4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; (8S,10S)-10-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione; (8S-cis)-10-((3-Amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroacetyl)-1-methoxy-5,12-naphthacenedione; (8S-cis)-10-[(3-Amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione; 1,2,3,4,6,11-hexahydro-4beta,5,12-trihydroxy-4-(hydroxyacetyl)-10-methoxy-6, 11-Dioxonaphthacen-1beta-yl-3-amino-2,3,6-trideoxy-alpha-l-lyxohexopyranoside; 1392315-46-6; 5,12-naphthacenedione, 10-((3-Amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione; 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)-
Click to Show/Hide
|
||||
| Structure |
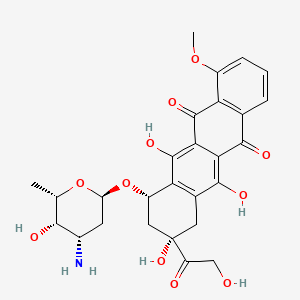 |
||||
| Formula |
C27H29NO11
|
||||
| IUPAC Name |
(7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione
|
||||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O
|
||||
| InChI |
InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1
|
||||
| InChIKey |
AOJJSUZBOXZQNB-TZSSRYMLSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Transferrin receptor protein 1 (TFRC)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor/Driver | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | N6-adenosine-methyltransferase non-catalytic subunit (METTL14) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor/Driver | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | KCNQ1OT1 (IncRNA) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor/Driver | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | hsa-miR-7-5p (miRNA) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| Response regulation | ENPP2 was transcriptionally regulated by FoxO4 to protect cardiomyocytes from doxorubicin-induced cardiotoxicity by inhibiting ferroptosis. In addition, the inhibitory effects of ENPP2 on Dox-induced ferroptosis were significantly reduced by FoxO4 overexpression, as demonstrated by increased Fe2+ and lipid ROS activity levels, decreased SLC7A11, GPX4 and FPN1 expression, and increased NOX4 expression, which were observed following FoxO4 overexpression. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
Male C57BL/6J mice were housed in a temperature- and humidity-controlled room, fed a commercial diet (CRF-1; Oriental Yeast Co. Ltd.), and given free access to water. GPx4 Tg mice and GPx4 hetKO mice were produced as previously described. In these gene-manipulated mice, GPx4 was systemically overexpressed or absent, respectively. These strains were backcrossed with C57BL/6J mice in our laboratory. The DIC model was reproduced as previously reported, with some modification. Briefly, DOX (6 mg/kg, body weight) was administered to mice via tail vein at days 0, 2, and 4.
Click to Show/Hide
|
||||
| Response regulation | Doxorubicin (DOX) downregulated glutathione peroxidase 4 (GPx4) and induced excessive lipid peroxidation through DOX-Fe2+ complex in mitochondria, leading to mitochondria-dependent ferroptosis. The findings suggest that mitochondria-dependent ferroptosis plays a key role in progression of doxorubicin-induced cardiomyopathy (DIC) and that ferroptosis is the major form of regulated cell death in DOX cardiotoxicity. | ||||
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | Acyl-coenzyme A thioesterase 1 (ACOT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
C57BL/6 male mice (20-25 g) at 7 weeks were purchased from the Vital River Laboratory Animal Technology Co., Ltd. Two doses of DOX was administrated by intraperitoneal injection, 15 mg/kg at Day 1, and 10 mg/kg at Day 8. Mice were then killed at Day 15 after transthoracic echocardiography examination.
Click to Show/Hide
|
||||
| Response regulation | Both in vitro and in vivo experiments proved the downregulation of Acot1 in doxorubicin-induced cardiotoxicity (DIC), which can be partially prevented with Fer-1 treatment. Acot1 may become a potential treating target in preventing doxorubicin-induced cardiotoxicity by anti-ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | N6-adenosine-methyltransferase non-catalytic subunit (METTL14) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response regulation | The RNA-binding protein IGF2BP1 is associated with KCNQ1OT1 to increase its stability and robustly inhibit miR-7-5p activity. MiR-7-5p could effectively suppress METLL14 and TFRC expression. The study suggested a therapeutic strategy to alleviate doxorubicin (DOX)-induced cardiomyopathy. | ||||
References
