Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10007)
| Target Name | Polyunsaturated fatty acid lipoxygenase ALOX12 (ALOX12) | ||||
|---|---|---|---|---|---|
| Synonyms |
Arachidonate (12S)-lipoxygenase ; Arachidonate (15S)-lipoxygenase ; Linoleate (13S)-lipoxygenase; Lipoxin synthase 12-LO; Platelet-type lipoxygenase 12
Click to Show/Hide
|
||||
| Gene Name | ALOX12 | ||||
| Sequence |
MGRYRIRVATGAWLFSGSYNRVQLWLVGTRGEAELELQLRPARGEEEEFDHDVAEDLGLL
QFVRLRKHHWLVDDAWFCDRITVQGPGACAEVAFPCYRWVQGEDILSLPEGTARLPGDNA LDMFQKHREKELKDRQQIYCWATWKEGLPLTIAADRKDDLPPNMRFHEEKRLDFEWTLKA GALEMALKRVYTLLSSWNCLEDFDQIFWGQKSALAEKVRQCWQDDELFSYQFLNGANPML LRRSTSLPSRLVLPSGMEELQAQLEKELQNGSLFEADFILLDGIPANVIRGEKQYLAAPL VMLKMEPNGKLQPMVIQIQPPNPSSPTPTLFLPSDPPLAWLLAKSWVRNSDFQLHEIQYH LLNTHLVAEVIAVATMRCLPGLHPIFKFLIPHIRYTMEINTRARTQLISDGGIFDKAVST GGGGHVQLLRRAAAQLTYCSLCPPDDLADRGLLGLPGALYAHDALRLWEIIARYVEGIVH LFYQRDDIVKGDPELQAWCREITEVGLCQAQDRGFPVSFQSQSQLCHFLTMCVFTCTAQH AAINQGQLDWYAWVPNAPCTMRMPPPTTKEDVTMATVMGSLPDVRQACLQMAISWHLSRR QPDMVPLGHHKEKYFSGPKPKAVLNQFRTDLEKLEKEITARNEQLDWPYEYLKPSCIENS VTI Click to Show/Hide
|
||||
| Family | Lipoxygenase family | ||||
| Function |
Catalyzes the regio and stereo-specific incorporation of molecular oxygen into free and esterified polyunsaturated fatty acids generating lipid hydroperoxides that can be further reduced to the corresponding hydroxy species. Mainly converts arachidonate ((5Z,8Z,11Z,14Z)-eicosatetraenoate) to the specific bioactive lipid (12S)-hydroperoxyeicosatetraenoate/(12S)-HPETE. Through the production of bioactive lipids like (12S)- HPETE it regulates different biological processes including platelet activation . It can also catalyze the epoxidation of double bonds of polyunsaturated fatty acids such as (14S)-hydroperoxy-docosahexaenoate/(14S)-HPDHA resulting in the formation of (13S,14S)-epoxy-DHA. Furthermore, it may participate in the sequential oxidations of DHA ((4Z,7Z,10Z,13Z,16Z,19Z)-docosahexaenoate) to generate specialized pro- resolving mediators (SPMs) like resolvin D5 ((7S,17S)-diHPDHA) and (7S,14S)-diHPDHA, that actively down-regulate the immune response and have anti-aggregation properties with platelets. An additional function involves a multistep process by which it transforms leukotriene A4/LTA4 into the bioactive lipids lipoxin A4/LXA4 and lipoxin B4/LXB4, both are vasoactive and LXA4 may regulate neutrophil function via occupancy of specific recognition sites. Can also peroxidize linoleate ((9Z,12Z)-octadecadienoate) to (13S)- hydroperoxyoctadecadienoate/ (13S-HPODE). Due to its role in regulating both the expression of the vascular endothelial growth factor (VEGF, an angiogenic factor involved in the survival and metastasis of solid tumors) and the expression of integrin beta-1 (known to affect tumor cell migration and proliferation), it can be regarded as protumorigenic. Important for cell survival, as it may play a role not only in proliferation but also in the prevention of apoptosis in vascular smooth muscle cells.
Click to Show/Hide
|
||||
| Gene ID | 239 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
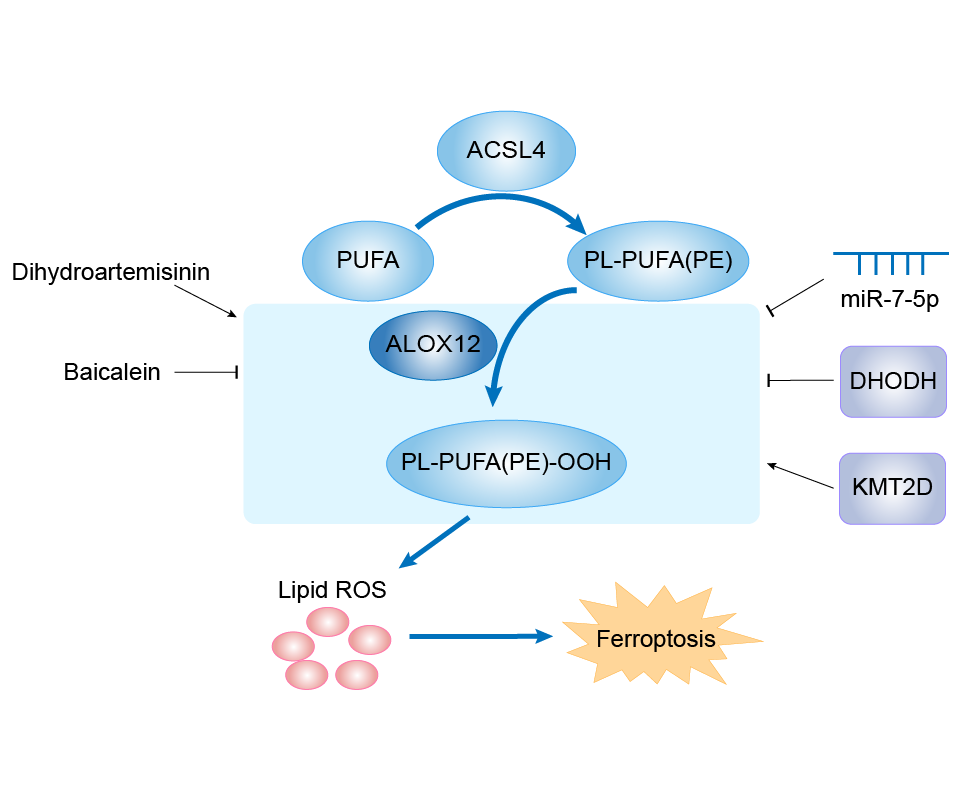
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
ALOX12 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
hsa-miR-7-5p (miRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Knockdown of miR-7-5p increased reactive oxygen species (ROS), mitochondrial membrane potential, and intracellular Fe2+amount. Furthermore, miR-7-5p knockdown results in the down-regulation of the iron storage gene expression such as ferritin, up-regulation of the ferroptosis marker ALOX12 gene expression, and increases of Liperfluo amount in Endocervical adenocarcinoma. | |||
Dihydroorotate dehydrogenase (quinone), mitochondrial (DHODH)
Anemia [ICD-11: 3A9Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AHH-1 cells | Normal | Homo sapiens | CVCL_3640 | |
| In Vivo Model |
4-week male C57BL/6J mice (20-22 g) were obtained from the vital river and preconditioned for one week. Mice were divided into two groups, either exposed to benzene or fresh air in the exposure system for 6 h/day, 6 days/week, for two months. In the last exposure day, the mice were fast for 10 h. Subsequently, the mice were injected with tribromoethanol (T48402, Sigma, USA) (300 mg/kg).
Click to Show/Hide
|
||||
| Response Description | The iron-regulatory system IRP1, ferroptosis regulator DHODH, and fatty acids metabolism rate-limiting enzyme ALOX12 were the crucial influencers in regulating ferroptosis, this provides the potential target in attenuating benzene-induced anemia of inflammation. | ||||
Unspecific Regulator
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| In Vivo Model |
Six to eight-week-old female C57BL/6 mice were purchased from the Experimental Animal Center of Military Medical Sciences (Beijing, China). C57BL/6 mice were anesthetized and the tail of the pancreas was exposed. Panc 02 cells were resuspended in PBS at a concentration of 1 x 106 cells/0.1 ml and 50 ul cells were injected into the tail of the pancreas. Tumor-bearing mice were randomly divided into two groups (3 days after implantation). The control group was intraperitoneally injected 200 ul PBS daily for 10 days, and the DHA group was intraperitoneally injected with 100 mg/kg DHA daily for 10 days. The pancreatic tumors and spleens of the mice were collected for subsequent analysis.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin has anti-tumor effect in pancreatic cancer cells in vitro and in vivo. DHA treatment induced ferroptosis by increasing P53 and AOLX12 expression. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Responsed Drug | Paraquat | Investigative | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All adult male C57/BL6 mice weighing 18-22g were obtained from the Experimental Animal Center of Central South University, China. Animals were randomly divided into six groups as follows: 1) control group (n = 6) was given vehicle intracranial injection (PBS 5 ul); 2) FeCl3 group (n = 6) was given 5 ul 50 mM FeCl3; 3) and 4) baicalein groups were pretreated with baicalein 50 mg/kg (n = 6) and 100 mg/kg (n = 6) 30 min prior to FeCl3 administration, respectively; 5) ferroptosis inhibitor group (n = 6) was administered continuously with 10 mg/kg Lipo-1 3 d prior to FeCl3 administration; and 6) baicalein administration group (n = 6) was given 100 mg/kg baicalein once by intraperitoneal injection 30 min prior to 5 ul PBS administration.
Click to Show/Hide
|
||||
| Response Description | Baicalein, as a naturel bioactive compound, could ameliorate behavioral seizures and play a key neuroprotective role in FeCl3-induced posttraumatic epileptic seizures through inhibiting ferroptosis and its neuroprotection might be related to suppression of 12-LOX/15-LOX. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | |||
| Responsed Drug | Hydrogen Sulfide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
C2C12 cells | Normal | Mus musculus | CVCL_0188 |
| Response Description | RSL3 induced ROS generation, inhibited CSE/H2S (Hydrogen sulfide) system, damaged mitochondrial structure, increased acetyl-CoA content and lipid peroxidation, which eventually lead to ferroptosis. Supplement of H2S normalized oxidative stress, acetyl-CoA content and ALOX12 acetylation and then protected myoblasts from RSL3-induced ferroptosis. | |||
Histone-lysine N-methyltransferase 2D (KMT2D)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell differentiation | |||||
In Vitro Model |
NHEK/SV3 cells | Normal | Homo sapiens | CVCL_9Q50 | |
| 3T3-J2 cells | Normal | Mus musculus | CVCL_W667 | ||
| In Vivo Model |
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Mice were maintained on a mixed C57BL/6 background on a standard light-dark cycle. Mice carrying Mll4SET floxed alleles, Mll3SET floxed alleles, or a combination of both of these were crossed with Krt14-Cre transgenic mice.
Click to Show/Hide
|
||||
| Response Description | MLL4 (KMT2D) deficiency profoundly alters epidermal gene expression and uniquely rewires the expression of key genes and markers of ferroptosis (Alox12, Alox12b, and Aloxe3). | ||||
Unspecific Regulator
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| In Vivo Model |
Six to eight-week-old female C57BL/6 mice were purchased from the Experimental Animal Center of Military Medical Sciences (Beijing, China). C57BL/6 mice were anesthetized and the tail of the pancreas was exposed. Panc 02 cells were resuspended in PBS at a concentration of 1 x 106 cells/0.1 ml and 50 ul cells were injected into the tail of the pancreas. Tumor-bearing mice were randomly divided into two groups (3 days after implantation). The control group was intraperitoneally injected 200 ul PBS daily for 10 days, and the DHA group was intraperitoneally injected with 100 mg/kg DHA daily for 10 days. The pancreatic tumors and spleens of the mice were collected for subsequent analysis.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin has anti-tumor effect in pancreatic cancer cells in vitro and in vivo. DHA treatment induced ferroptosis by increasing P53 and AOLX12 expression. | ||||
Paraquat
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Baicalein
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All adult male C57/BL6 mice weighing 18-22g were obtained from the Experimental Animal Center of Central South University, China. Animals were randomly divided into six groups as follows: 1) control group (n = 6) was given vehicle intracranial injection (PBS 5 ul); 2) FeCl3 group (n = 6) was given 5 ul 50 mM FeCl3; 3) and 4) baicalein groups were pretreated with baicalein 50 mg/kg (n = 6) and 100 mg/kg (n = 6) 30 min prior to FeCl3 administration, respectively; 5) ferroptosis inhibitor group (n = 6) was administered continuously with 10 mg/kg Lipo-1 3 d prior to FeCl3 administration; and 6) baicalein administration group (n = 6) was given 100 mg/kg baicalein once by intraperitoneal injection 30 min prior to 5 ul PBS administration.
Click to Show/Hide
|
||||
| Response Description | Baicalein, as a naturel bioactive compound, could ameliorate behavioral seizures and play a key neuroprotective role in FeCl3-induced posttraumatic epileptic seizures through inhibiting ferroptosis and its neuroprotection might be related to suppression of 12-LOX/15-LOX. | ||||
Hydrogen Sulfide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | C2C12 cells | Normal | Mus musculus | CVCL_0188 |
| Response Description | RSL3 induced ROS generation, inhibited CSE/H2S (Hydrogen sulfide) system, damaged mitochondrial structure, increased acetyl-CoA content and lipid peroxidation, which eventually lead to ferroptosis. Supplement of H2S normalized oxidative stress, acetyl-CoA content and ALOX12 acetylation and then protected myoblasts from RSL3-induced ferroptosis. | |||
References
