Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0169)
| Name |
Triptolide
|
||||
|---|---|---|---|---|---|
| Synonyms |
triptolide; 38748-32-2; Triptolid; PG490; NSC 163062; NSC-163062; 19ALD1S53J; CHEBI:9747; CHEMBL463763; PG-490; (1S,2S,4S,5S,7R,8R,9S,11S,13S)-8-hydroxy-1-methyl-7-propan-2-yl-3,6,10,16-tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18]icos-14(18)-en-17-one; (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-6-hydroxy-8b-methyl-6a-(propan-2-yl)-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydrotrisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one; (5bS,6aS,7aS,8R,8aR,9aS,9bS,10aS,10bS)-8-Hydroxy-8a-isopropyl-10b-methyl-1,5,5b,6,6a,8,8a,9a,9b,10b-decahydrotris(oxireno)[2',3':4b,5;2'',3'':6,7;2''',3''':8a,9]phenanthro[1,2-c]furan-3(2H)-one; (6aS,7aS,8R,8aR,9aS,9bS,10aS,10bS)-8-hydroxy-8a-isopropyl-10b-methyl-1,5,5b,6,6a,8,8a,9a,9b,10b-decahydrotris(oxireno)[2',3':4b,5;2'',3'':6,7;2''',3''':8a,9]phenanthro[1,2-c]furan-3(2H)-one.; SMR000466307; UNII-19ALD1S53J; NSC163062; Triptolide, 1; (3BS,4AS,5AS,6R,6AR,7AS,7BS,8AS,8BS)-3B,4,4A,6,6A,7A,7B,8B,9,10-DECAHYDRO-6-HYDROXY-8B-METHYL-6A-(1-METHYLETHYL)TRISOXIRENO(4B,5:6,7:8A,9)PHENANTHRO(1,2-C)FURAN-1(3H)-ONE; (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-3b,4,4a,6,6a,7a,7b,8b,9,10-Decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)trisoxireno[4b,5:6,7:8a,9]phenanthro[1,2-c]furan-1(3H)-one; Trisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-; MFCD00210565; CPD000466307; TRIPTOLIDE [MI]; TRIPTOLIDE [WHO-DD]; BSPBio_001595; KBioGR_000315; KBioSS_000315; MLS000759410; MLS001424107; MLS006010844; SCHEMBL413634; DTXSID5041144; EX-A7744A; KBio2_000315; KBio2_002883; KBio2_005451; KBio3_000629; KBio3_000630; DFBIRQPKNDILPW-CIVMWXNOSA-N; Bio2_000315; Bio2_000795; HMS1361P17; HMS1791P17; HMS1989P17; HMS2051N13; HMS3402P17; 144539-79-7; TPL; BDBM50241049; NSC839303; s3604; AKOS022168197; AM84923; CCG-100957; CS-0286; DB12025; NC00207; NSC-839303; IDI1_034065; NCGC00163411-01; NCGC00163411-02; NCGC00163411-03; NCGC00163411-07; BP-25386; BS-16697; HY-32735; NCI60_001223; Trisoxireno(4b,5:6,7:8a,9)phenanthro(1,2-c)furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-; T2899; C09204; AB00639938-06; AB00639938-08; Q906351; Q-100450; BRD-K39484304-001-02-5; BRD-K39484304-001-06-6; BRD-K39484304-001-16-5; Triptolide, Tripterygium wilfordii - CAS 38748-32-2; (1S,2S,4S,5S,7R,8R,9S,11S)-8-Hydroxy-1-methyl-7-propan-2-yl-3,6,10,16-tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18]icos-14(18)-en-17-one; (3BS,4AS,5AS,6R,6AR,7AS,7BS,8AS,8BS)-3B,4,4A,6,6A,7A,7B,8B,9,10-DECAHYDRO-6-HYDROXY-6A-ISOPROPYL-8B-METHYLTRISOXIRENO(6,7:8A,9:4B,5)PHENANTHRO(1,2-C)FURAN-1(3H)-ONE; (5bS,6aS,7aS,8R,8aR,9aS,9bS,10aS,10bS)-8-hydroxy-8a-isopropyl-10b-methyl-2,5,5b,6,6a,8,8a,9a,9b,10b-decahydrotris(oxireno)[2',3':4b,5;2'',3'':6,7;2''',3''':8a,9]phenanthro[1,2-c]furan-3(1H)-one; Trisoxireno[4b,7:8a,9]phenanthro[1,2-c]furan-(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, [3bR-(3b.alpha.,4a.alpha.,5aS*,6.beta.,6a.beta.,7a.beta.,7b.alpha.,8aS*,8b.beta.)]-; Trisoxireno[4b,7:8a,9]phenanthro[1,2-c]furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, [3bR-(3b.alpha.,4a.alpha.,5aS*,6.beta.,6a.beta.,7a.beta.,7b.alpha.,8aS*,8b.beta.)]-
Click to Show/Hide
|
||||
| Structure |
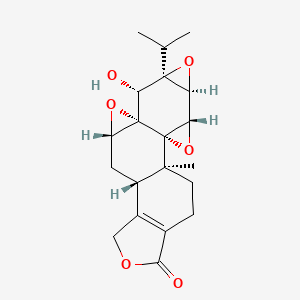 |
||||
| Formula |
C20H24O6
|
||||
| IUPAC Name |
(1S,2S,4S,5S,7R,8R,9S,11S,13S)-8-hydroxy-1-methyl-7-propan-2-yl-3,6,10,16-tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18]icos-14(18)-en-17-one
|
||||
| Canonical SMILES |
CC(C)C12C(O1)C3C4(O3)C5(CCC6=C(C5CC7C4(C2O)O7)COC6=O)C
|
||||
| InChI |
InChI=1S/C20H24O6/c1-8(2)18-13(25-18)14-20(26-14)17(3)5-4-9-10(7-23-15(9)21)11(17)6-12-19(20,24-12)16(18)22/h8,11-14,16,22H,4-7H2,1-3H3/t11-,12-,13-,14-,16+,17-,18-,19+,20+/m0/s1
|
||||
| InChIKey |
DFBIRQPKNDILPW-CIVMWXNOSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ovarian cancer | ICD-11: 2C73 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A2780/DDP cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_D619 | |
| In Vivo Model |
All female BALB/cnude mice(4-6 weeks old, 15-20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). They were raised in specific pathogen-free conditions and allowed to access sterile water and food freely. A2780/DDP cell suspension (100 uL) with a density of 1 x 107 cells/mL was injected subcutaneously into the axilla of the mice. After observing the nude mice for a week, it was confirmed that subcutaneous A2780/DDP cells were inoculated successfully. Sterile saline (100 uL) was injected into the abdominal cavity of the nude mice in the control group for 14 days. The mice in the DDP treatment group were given DDP (4 mg/kg/day) intraperitoneally on the first and eighth days. TG (100 uL, 1 mg/kg) diluted with sterile physiological saline were injected into the abdominal cavity of the nude mice in the TG treatment group for 14 days. In addition, the nude mice in the TG + DDP treatment group were given TG (100 uL, 1 mg/kg) for 14 days and DDP (4 mg/kg/day) intraperitoneally on the first and eighth days.
Click to Show/Hide
|
||||
| Response regulation | Tripterygium (TG) can effectively inhibit the proliferation of drug-resistant ovarian tumor cells A2780/DDP and increase the sensitivity to cisplatin chemotherapy both invitro and invivo. In terms of mechanism, TG induces ferroptosis by targeting the NRF2/GPX4 signal axis to weaken the antioxidant capacity of cancer cells. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Myeloid leukaemia | ICD-11: 2B33 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| Response regulation | Triptolide inhibited Nrf2 expression and induced leukemia cell ferroptosis, as evidenced by increased ROS levels and lipid oxidation as well as decreased glutathione peroxidase 4 expression. Therefore, it is plausible that triptolide can promote leukemia cell sensitivity to DOX via downregulation of Nrf2 expression. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Ovarian cancer | ICD-11: 2C73 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A2780/DDP cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_D619 | |
| In Vivo Model |
All female BALB/cnude mice(4-6 weeks old, 15-20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). They were raised in specific pathogen-free conditions and allowed to access sterile water and food freely. A2780/DDP cell suspension (100 uL) with a density of 1 x 107 cells/mL was injected subcutaneously into the axilla of the mice. After observing the nude mice for a week, it was confirmed that subcutaneous A2780/DDP cells were inoculated successfully. Sterile saline (100 uL) was injected into the abdominal cavity of the nude mice in the control group for 14 days. The mice in the DDP treatment group were given DDP (4 mg/kg/day) intraperitoneally on the first and eighth days. TG (100 uL, 1 mg/kg) diluted with sterile physiological saline were injected into the abdominal cavity of the nude mice in the TG treatment group for 14 days. In addition, the nude mice in the TG + DDP treatment group were given TG (100 uL, 1 mg/kg) for 14 days and DDP (4 mg/kg/day) intraperitoneally on the first and eighth days.
Click to Show/Hide
|
||||
| Response regulation | Tripterygium (TG) can effectively inhibit the proliferation of drug-resistant ovarian tumor cells A2780/DDP and increase the sensitivity to cisplatin chemotherapy both invitro and invivo. In terms of mechanism, TG induces ferroptosis by targeting the NRF2/GPX4 signal axis to weaken the antioxidant capacity of cancer cells. | ||||
References
