Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0147)
| Name |
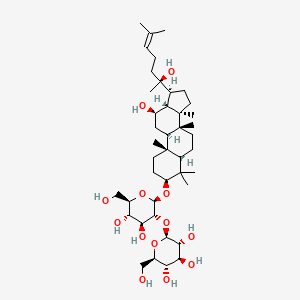
Ginsenoside Rg3
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ginsenoside Rg3; 14197-60-5; 20(S)-Ginsenoside Rg3; (20S)-Propanaxadiol; 20s-ginsenoside rg3; 20S-propanaxadiol; (20S)-ginsenoside Rg3; S-Ginsenoside Rg3; 20(S)-Ginsenoside-Rg3; 20(S)-Propanaxidiol; CHEMBL398412; CHEBI:67991; 227D367Y57; (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol; 11019-45-7; Rg3; ginsenoside 20-rg3; ginsenoside rg3, (s)-; ginsenoside rg3, (+)-; 20(S)-Propanaxadiol;S-ginsenoside Rg3; UNII-227D367Y57; MFCD06410950; (R)Ginsenoside-Rg3; Ginsenoside Rg3,(S); GTPL7658; GINSENOSIDE RG3 [WHO-DD]; RWXIFXNRCLMQCD-JBVRGBGGSA-N; DTXSID101316982; GLXC-19139; HMS3886B14; BDBM50317537; Ginsenoside Rg3, analytical standard; s9022; Ginsenoside Rg3, >=98% (HPLC); AKOS037514674; CCG-270472; (3beta,12beta)-12,20-dihydroxydammar-24-en-3-yl 2-O-beta-D-glucopyranosyl-beta-D-glucopyranoside; AS-56617; Dammar-24-ene-12-beta,20-diol, 3-beta-((2-O-beta-D-glucopyranosyl-beta-D-glucopyransoyl)oxy)-; C20778; Q-100154; Q27077807; .BETA.-D-GLUCOPYRANOSIDE, (3.BETA.,12.BETA.)-12,20-DIHYDROXYDAMMAR-24-EN-3-YL 2-O-.BETA.-D-GLUCOPYRANOSYL-; 3-O-.BETA.-D-GLUCOPYRANOSYL-(1->2)-.BETA.-D-GLUCOPYRANOSYLDAMMAR-24-ENE-3.BETA.,12.BETA.,20S-TRIOL; dammar-24-ene-12beta,20-diol, 3-beta-((2-O-beta-D-glucopyranosyl-beta-D-glucopyransoyl)oxy)-
Click to Show/Hide
|
||||
| Structure |
 |
||||
|
3D MOL
|
|||||
| Formula |
C42H72O13
|
||||
| IUPAC Name |
(2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
|
||||
| Canonical SMILES |
CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)CO)O)O)O)C)C)O)C)O)C
|
||||
| InChI |
InChI=1S/C42H72O13/c1-21(2)10-9-14-42(8,51)22-11-16-41(7)29(22)23(45)18-27-39(5)15-13-28(38(3,4)26(39)12-17-40(27,41)6)54-37-35(33(49)31(47)25(20-44)53-37)55-36-34(50)32(48)30(46)24(19-43)52-36/h10,22-37,43-51H,9,11-20H2,1-8H3/t22-,23+,24+,25+,26-,27+,28-,29-,30+,31+,32-,33-,34+,35+,36-,37-,39-,40+,41+,42-/m0/s1
|
||||
| InChIKey |
RWXIFXNRCLMQCD-JBVRGBGGSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Acute pancreatitis | ICD-11: DC31 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks old; SPF; weighing 24-26 g, n = 16 in total) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All mice were housed in an environmentally controlled room at a temperature ranging from 20 to 24 on a 12 h light/dark cycle and used in the experiments following an overnight fast with water, availablead libitum. All procedures followed the Principles of Laboratory Animal Care (NIH publication number 85Y23, revised in 1996), and the experimental protocol was approved by the Animal Care Committee, Nanjing Medical University (NMU-2021JK-085).
Click to Show/Hide
|
||||
| Response regulation | Taken together, the present study, to the best of our knowledge, is the first to reveal a protective role for Ginsenoside Rg3 in mice with acute pancreatitis by suppressing oxidative stressrelated ferroptosis and the activation of the NRF2/HO1 pathway. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute pancreatitis | ICD-11: DC31 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks old; SPF; weighing 24-26 g, n = 16 in total) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All mice were housed in an environmentally controlled room at a temperature ranging from 20 to 24 on a 12 h light/dark cycle and used in the experiments following an overnight fast with water, availablead libitum. All procedures followed the Principles of Laboratory Animal Care (NIH publication number 85Y23, revised in 1996), and the experimental protocol was approved by the Animal Care Committee, Nanjing Medical University (NMU-2021JK-085).
Click to Show/Hide
|
||||
| Response regulation | Taken together, the present study, to the best of our knowledge, is the first to reveal a protective role for Ginsenoside Rg3 in mice with acute pancreatitis by suppressing oxidative stressrelated ferroptosis and the activation of the NRF2/HO1 pathway. | ||||
