Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0132)
| Name |
Camptothecin
|
||||
|---|---|---|---|---|---|
| Synonyms |
camptothecin; Camptothecine; 7689-03-4; (S)-(+)-Camptothecin; Campathecin; (+)-Camptothecine; d-Camptothecin; (+)-Camptothecin; 20(S)-Camptothecine; 21,22-Secocamptothecin-21-oic acid lactone; NSC94600; Camptothecine (S,+); CHEMBL65; (S)-4-ethyl-4-hydroxy-1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; NSC-94600; (4S)-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; MLS000766223; XT3Z54Z28A; CHEBI:27656; MFCD00081076; (19S)-19-ethyl-19-hydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaene-14,18-dione; (19S)-19-ethyl-19-hydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione; (S)-Camptothecin; 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (4S)-; 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (S)-; 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (4S)-; 20(S)-Camptothecin; 4-ETHYL-4-HYDROXY-1,12-DIHYDRO-4H-2-OXA-6,12A-DIAZA-DIBENZO[B,H]FLUORENE-3,13-DIONE; SR-01000075798; SR-01000597379; d-camptothecine; (s)-camptothecine; Camptothecin,(S); (4S)-4-ETHYL-4-HYDROXY-1H-PYRANO(3',4':6,7)INDOLIZINO(1,2-B)QUINOLINE-3,14(4H,12H)-DIONE; (S)-4-ethyl-4-hydroxy-1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione; (S)-4-Ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-(4H,12H)-dione; 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (S)-; Prestwick_102; (+)-Camptothecin;; Camptothecine (8CI); Spectrum_000299; Tocris-1100; SpecPlus_000712; Prestwick0_000200; Prestwick1_000200; Prestwick2_000200; Prestwick3_000200; Spectrum2_000903; Spectrum3_001203; Spectrum4_000738; Spectrum5_001126; CAMPTOTHECIN [MI]; Lopac-C-9911; SCHEMBL6038; UNII-XT3Z54Z28A; Lopac0_000341; BSPBio_000159; BSPBio_002586; KBioGR_001036; KBioSS_000779; KBioSS_002283; cid_24360; CAMPTOTHECIN [WHO-DD]; DivK1c_000826; DivK1c_006808; SPECTRUM1502232; SPBio_000746; SPBio_002080; BPBio1_000175; DTXSID0030956; HMS502J08; KBio1_000826; KBio1_001752; KBio2_000779; KBio2_003347; KBio2_005915; KBio3_002086; 4-Ethyl-4-hydroxy-1H-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; NINDS_000826; Bio1_000400; Bio1_000889; Bio1_001378; HMS1568H21; HMS1921N08; HMS2089F08; HMS2095H21; HMS3261E03; HMS3414J17; HMS3654D13; HMS3678J15; HMS3712H21; BCP02857; Tox21_500341; AC-202; BBL033963; BDBM50008923; CCG-40255; GR-301; s1288; STK801886; AKOS004119861; CS-1049; DB04690; KS-5235; LP00341; SDCCGMLS-0066688.P001; SDCCGSBI-0050329.P003; CAS-2114454; IDI1_000826; NCGC00015290-01; NCGC00016994-01; NCGC00016994-02; NCGC00016994-03; NCGC00016994-04; NCGC00016994-05; NCGC00016994-06; NCGC00016994-07; NCGC00016994-08; NCGC00016994-09; NCGC00016994-10; NCGC00016994-11; NCGC00016994-12; NCGC00016994-16; NCGC00016994-29; NCGC00024997-01; NCGC00024997-02; NCGC00024997-03; NCGC00024997-04; NCGC00024997-05; NCGC00024997-06; NCGC00178592-01; NCGC00178592-02; NCGC00261026-01; HY-16560; NCI60_042105; SMR000445686; SY010324; EU-0100341; SW196414-3; C 9911; C01897; M01564; AB00052452-08; AB00052452-09; AB00052452_10; EN300-1725804; (S)-(+)-Camptothecin, >=90% (HPLC), powder; A838882; Q419964; Q-200785; SR-01000075798-1; SR-01000075798-4; SR-01000597379-1; SR-01000597379-3; BRD-K37890730-001-09-4; BRD-K37890730-001-10-2; Z1741982070; (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]florene-3,13-dione; (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione; 4-Ethyl-4-hydroxy-1H-pyrano-[3[,4[:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; (19S)-19-ethyl-19-hydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaene-14,18-dione; (S)-4-Ethyl-4-hydroxy-1H-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; (S)-4-Ethyl-4-hydroxy-1H-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione;(S)-(+)-Camptothecin; (S)-4-Ethyl-4-hydroxy-1H-pyrano[3 inverted exclamation mark ,4 inverted exclamation mark :6,7]indolizino[1,2-b]quinoline-3,14-(4H,12H)-dione; 1H-Pyrano[3',7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-4-hydroxy-, (S)-; 4(S)-Ethyl-4-hydroxy-1H-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14 (4H,12H)-dione; 4-ethyl-4-hydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3'',4'':6,7]indolizino[1,2-b]quinoline-3,14-dione; 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin or CPT); 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (Camptothecin); 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (CPT, Camptothecin)
Click to Show/Hide
|
||||
| Structure |
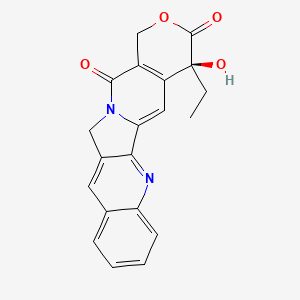 |
||||
| Formula |
C20H16N2O4
|
||||
| IUPAC Name |
(19S)-19-ethyl-19-hydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaene-14,18-dione
|
||||
| Canonical SMILES |
CCC1(C2=C(COC1=O)C(=O)N3CC4=CC5=CC=CC=C5N=C4C3=C2)O
|
||||
| InChI |
InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
|
||||
| InChIKey |
VSJKWCGYPAHWDS-FQEVSTJZSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response regulation | Sorafenib is a potent inducer of ferroptosis used to manage hepatocellular carcinoma (HCC). Nrf2 inhibition by Camptothecin improves sorafenib's sensitivity and reduces sorafenib's resistance via the augmentation of sorafenib's ferroptosis action. | |||
