Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0195)
| Name |
lipoxin A4
|
||||
|---|---|---|---|---|---|
| Synonyms |
lipoxin A4; LXA4; 89663-86-5; 5S,6R-LipoxinA4; CHEBI:6498; 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-Eicosatetraenoic acid; F7C6J3D79J; LIPOXINA4; (5S,6R,7E,9E,11Z,13E,15S)-5,6,15-trihydroxyicosa-7,9,11,13-tetraenoic acid; (7E,9E,11Z,13E)-(5S,6R,15S)-5,6,15-Trihydroxyicosa-7,9,11,13-tetraenoic acid; Lipoxin A; 5,6,15-triHETE; 7,9,11,13-Eicosatetraenoicacid, 5,6,15-trihydroxy-, (5S,6R,7E,9E,11Z,13E,15S)-; 5S,6S-Lipoxin A4; LXA4-[19,19,20,20,20-d5]; UNII-F7C6J3D79J; 6R-LXA4; BSPBio_001378; BML1-E11; CHEMBL392438; GTPL1034; DTXSID6040535; HMS1361E20; HMS1791E20; HMS1989E20; HMS3402E20; BDBM50520816; LMFA03040001; AKOS040755014; IDI1_033848; NCGC00161280-01; NCGC00161280-02; NCGC00161280-03; HY-113509; CS-0062443; C06314; SR-01000946984; SR-01000946984-1; Q27082450; 5S,6R,15S-Trihydroxy-7E,9E,11Z,13E-eicosatetraenoate; (5S,6R,15S)-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid; 5(S),6(R),15(S)-trihydroxyeicosa-7E,9E,11Z,13E-tetraenoic acid; 5S,6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid; (5S,6R,7E,9E,11Z,13E,15S)-5,6,15-trihydroxy-7,9,11,13-eicosatetraenoic acid; (5S,6R,7E,9E,11Z,13E,15S)-5,6,15-trihydroxyeicosa-7,9,11,13-tetraenoic acid; (7E,9E,11Z,13E)-(5S,6R,15S)-5,6,15-Trihydroxyicosa-7,9,11,13-tetraenoate; 7,9,11,13-Eicosatetraenoic acid, 5,6,15-trihydroxy-, (5S,6R,7E,9E,11Z,13E,15S)-
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
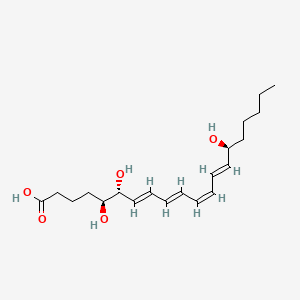 |
||||
| Formula |
C20H32O5
|
||||
| IUPAC Name |
(5S,6R,7E,9E,11Z,13E,15S)-5,6,15-trihydroxyicosa-7,9,11,13-tetraenoic acid
|
||||
| Canonical SMILES |
CCCCCC(C=CC=CC=CC=CC(C(CCCC(=O)O)O)O)O
|
||||
| InChI |
InChI=1S/C20H32O5/c1-2-3-8-12-17(21)13-9-6-4-5-7-10-14-18(22)19(23)15-11-16-20(24)25/h4-7,9-10,13-14,17-19,21-23H,2-3,8,11-12,15-16H2,1H3,(H,24,25)/b6-4-,7-5+,13-9+,14-10+/t17-,18+,19-/m0/s1
|
||||
| InChIKey |
IXAQOQZEOGMIQS-SSQFXEBMSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Spinal cord injury | ICD-11: ND51 | |||
| Responsed Regulator | RAC-alpha serine/threonine-protein kinase (AKT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPSCNs (Mouse primary spinal cord neurons) | ||||
| In Vivo Model |
Pregnant C57BL/6 mouse was purchased from Laboratory Animal Center of Xinxiang Medical University. pregnant mouse was anesthetized with CO2 and sacrificed by cervical dislocation at embryonic day 15. All embryos were separated from pregnant mouse under aseptic conditions. Under dissection microscope, each embryo was quickly killed by cervical dislocation, and the spinal cord was isolated. The membrane of the spinal cord and dorsal root ganglion was removed from the spinal cord applying microforceps. Subsequently, the spinal cord was quickly cut into small pieces (1 mm3) using ultrafine microscissors.
Click to Show/Hide
|
||||
| Response regulation | Lipoxin A4 (LXA4) enhanced the protein expression of p-AKT, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and haem-oxygenase-1 (HO-1) in primary spinal cord neurons. LXA4 exerted a neuroprotective effect in Erastin-induced ferroptosis of primary spinal cord neurons by activating the Akt/Nrf2/HO-1 signaling pathway. Thus, LXA4 may be a potential therapeutic agent for spinal cord injury (SCI). | ||||
