Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0054)
| Name |
Astaxanthin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Astaxanthin; 472-61-7; Ovoester; all-trans-Astaxanthin; AstaREAL; 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione; Astaxanthine; BioAstin; (3S,3'S)-Astaxanthin; trans-Astaxanthin; Astaxanthin, (3S,3'S)-; (3S,3'S)-all-trans-Astaxanthin; 8XPW32PR7I; 3,3'-Dihydroxy-beta-carotene-4,4'-dione; (3S,3'S)-3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione; CHEBI:40968; NSC-635689; (6S,6'S)-3,3'-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(6-hydroxy-2,4,4-trimethylcyclohex-2-enone); .beta.,.beta.-Carotene-4,4'-dione, 3,3'-dihydroxy-, (3S,3'S)-; Algae Haematococcus pluvialis; (6S)-6-hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohexen-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethylcyclohex-2-en-1-one; Natupink; AstaXin; Carophyll Pink; Lucantin Pink; BioAstin oleoresin; (6S)-6-Hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexenyl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethyl-1-cyclohex-2-enone; Astaxanthin (6CI); Astaxanthin, all-trans-; UNII-8XPW32PR7I; astaxantin; CCRIS 7118; HSDB 7468; EINECS 207-451-4; NSC 635689; 3S,3'S-Astaxanthin; Astaxanthin, all-trans-, (3S,3'S)-; Astaxanthin, 5% active; ASTAXANTHIN [MI]; b-Carotene-4,4'-dione; ASTAXANTHIN [HSDB]; ASTAXANTHIN [INCI]; ASTAXANTHIN [VANDF]; beta-Carotene-4,4'-dione; ASTAXANTHIN [MART.]; SCHEMBL20047; ASTAXANTHIN [USP-RS]; ASTAXANTHIN [WHO-DD]; E161j; CHEMBL1255871; Astaxanthin, >=98% (HPLC); E 161j; DTXSID00893777; all-trans-(3S,3'S)-astaxanthin; 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione, (3S,3'S)-; HMS3885C08; BCP05821; HY-B2163; LMPR01070263; MFCD00672621; s3834; AKOS015841055; AKOS015895756; AC-8760; BCP9000329; CCG-270185; DB06543; all-trans-Astaxanthin, analytical standard; AS-14095; CS-0020413; C08580; M01303; 3,3'-dihydroxy-4,4'-diketo-beta,beta-carotene; A827177; Q413740; Q-200655; 3,3'-DIHYDROXY-4,4'-DIKETO-.BETA.-CAROTENE; all-trans-3,3'-dihydroxy-b-Carotene-4,4'-dione (8CI); .beta.-Carotene-4,4'-dione, 3,3'-dihydroxy-, all-trans-; 3-(4,6-Dimethyl-2-oxo-2H-pyrimidin-1-yl)-propionicacid; all-trans-3,3'-dihydroxy-beta-Carotene-4,4'-dione (8CI); 3,3'-Dihydroxy-.beta.,.beta.-carotene-4,4'-dione, (3S,3'S)-; (3S,3'S)-3,3'-DIHYDROXY-.BETA.,.BETA.-CAROTENE-4,4'-DIONE; 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione;(S)-6-hydroxy-3-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-((S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohex-1-enyl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl)-2,4,4-trimethylcyclohex-2-enone;
Click to Show/Hide
|
||||
| Structure |
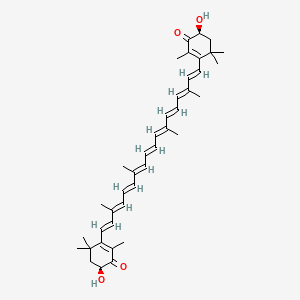 |
||||
| Formula |
C40H52O4
|
||||
| IUPAC Name |
(6S)-6-hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohexen-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethylcyclohex-2-en-1-one
|
||||
| Canonical SMILES |
CC1=C(C(CC(C1=O)O)(C)C)C=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=CC2=C(C(=O)C(CC2(C)C)O)C)C)C
|
||||
| InChI |
InChI=1S/C40H52O4/c1-27(17-13-19-29(3)21-23-33-31(5)37(43)35(41)25-39(33,7)8)15-11-12-16-28(2)18-14-20-30(4)22-24-34-32(6)38(44)36(42)26-40(34,9)10/h11-24,35-36,41-42H,25-26H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,27-15+,28-16+,29-19+,30-20+/t35-,36-/m0/s1
|
||||
| InChIKey |
MQZIGYBFDRPAKN-UWFIBFSHSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Lung injury | ICD-11: NB32 | |||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
6-week-Babl/c female mice were randomized to the following three groups of seven mice each: vehicle group, LPS group, Astaxanthin plus LPS group. Astaxanthin plus LPS group mice were pretreated with astaxanthin (20 mg/kg) byi.v injectionfor daily for 7 consecutive days. Astaxanthin was dissolved in 2%DMSO (vol/vol), 40% PEG-400 (vol/vol), 2% Tween 80 (vol/vol), and 56% PBS (vol/vol). On the last day, the mice were intraperitoneally injected with 5 mg/kg LPS or normal saline 2 h after the injection of astaxanthin. After 6 h of LPS stimulation, mice were euthanized to collect the BALF, and lung tissue samples. BALF was collected three times through a tracheal cannula with autoclaved normal saline, instilled up to a total volume of 1.8 ml.
Click to Show/Hide
|
||||
| Response regulation | Astaxanthin protected LPS-induced cell inflammation and acute lung injury (ALI) in mice by inhibiting ferroptosis, and its effect was achieved through Keap1-Nrf2/HO-1 pathway. Therefore, our study indicates that ferroptosis will become a new target for the treatment of ALI, and astaxanthin is a potential drug for the treatment of ALI. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Mice were randomly divided into four groups as follows (n = 5): (1) control, (2) APAP (MCE, Monmouth Junction, NJ, USA), (3) olive oil + APAP (oil + APAP), and (4) ASX (Energy Chemical, Shanghai, China) dissolved in olive oil + APAP (ASX + APAP). Astaxanthin was dissolved in olive oil to obtain a mixture of 20 mg/mL. Mice in groups 3 and 4 were given a dose of olive oil and a mixture of 5 mL/kgBW by gavage every day for 2 weeks. On day 15, mice in groups 2, 3, and 4 were given a peritoneal injection of 500 mg/kg APAP to induce liver injury. The mice were fasted for 12 h before the administration of APAP. Ten hours after APAP administration, blood and liver tissue were collected for further examination and analyses. Blood was centrifuged to obtain supernatants,which were stored at -80. Liver tissues were immediately removed from each animal, and homogenates were processed with formaldehyde and glutaraldehyde for protein and histological analysis.
Click to Show/Hide
|
||||
| Response regulation | Astaxanthin reduced inflammation through the NF-B pathway, inhibited oxidative stress and ferroptosis, and increased autophagy through the Nrf2/HO-1 pathway, ameliorating acetaminophen-induced liver injury in vivo and in vitro. | ||||
Heme oxygenase 1 (HMOX1)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung injury | ICD-11: NB32 | |||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
6-week-Babl/c female mice were randomized to the following three groups of seven mice each: vehicle group, LPS group, Astaxanthin plus LPS group. Astaxanthin plus LPS group mice were pretreated with astaxanthin (20 mg/kg) byi.v injectionfor daily for 7 consecutive days. Astaxanthin was dissolved in 2%DMSO (vol/vol), 40% PEG-400 (vol/vol), 2% Tween 80 (vol/vol), and 56% PBS (vol/vol). On the last day, the mice were intraperitoneally injected with 5 mg/kg LPS or normal saline 2 h after the injection of astaxanthin. After 6 h of LPS stimulation, mice were euthanized to collect the BALF, and lung tissue samples. BALF was collected three times through a tracheal cannula with autoclaved normal saline, instilled up to a total volume of 1.8 ml.
Click to Show/Hide
|
||||
| Response regulation | Astaxanthin protected LPS-induced cell inflammation and acute lung injury (ALI) in mice by inhibiting ferroptosis, and its effect was achieved through Keap1-Nrf2/HO-1 pathway. Therefore, our study indicates that ferroptosis will become a new target for the treatment of ALI, and astaxanthin is a potential drug for the treatment of ALI. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Mice were randomly divided into four groups as follows (n = 5): (1) control, (2) APAP (MCE, Monmouth Junction, NJ, USA), (3) olive oil + APAP (oil + APAP), and (4) ASX (Energy Chemical, Shanghai, China) dissolved in olive oil + APAP (ASX + APAP). Astaxanthin was dissolved in olive oil to obtain a mixture of 20 mg/mL. Mice in groups 3 and 4 were given a dose of olive oil and a mixture of 5 mL/kgBW by gavage every day for 2 weeks. On day 15, mice in groups 2, 3, and 4 were given a peritoneal injection of 500 mg/kg APAP to induce liver injury. The mice were fasted for 12 h before the administration of APAP. Ten hours after APAP administration, blood and liver tissue were collected for further examination and analyses. Blood was centrifuged to obtain supernatants,which were stored at -80. Liver tissues were immediately removed from each animal, and homogenates were processed with formaldehyde and glutaraldehyde for protein and histological analysis.
Click to Show/Hide
|
||||
| Response regulation | Astaxanthin reduced inflammation through the NF-B pathway, inhibited oxidative stress and ferroptosis, and increased autophagy through the Nrf2/HO-1 pathway, ameliorating acetaminophen-induced liver injury in vivo and in vitro. | ||||
References
