Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0278)
| Name |
ginkgolide-B
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ginkgolide B; ginkgolide-B; 15291-77-7; Ginkolide B; CHEMBL514432; BN 52021; BN-52021; SMR000544403; Gingkolide B; Ginkgolide B,(S); Ginkgolide B from Ginkgo biloba leaves; MLS001216216; MLS006011991; 18 - Potency of Gingko biloba; SCHEMBL12714280; Ginkgolide B, analytical standard; SQOJOAFXDQDRGF-MMQTXUMRSA-N; HMS2864N19; BDBM50251276; AKOS025311549; DB06744; G-170; Q-100180; Q27095719; Ginkgolide B from Ginkgo biloba leaves, >=90% (HPLC); ((1R,3R,6R,7S,8S,10R,11R,12R,13S,16S,17R)-8-tert-butyl-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclo[8.7.2.0^{1,11}.0^{3,7}.0^{7,11}.0^{13,17}]nonadecane-5,15,18-trione.
Click to Show/Hide
|
||||
| Structure |
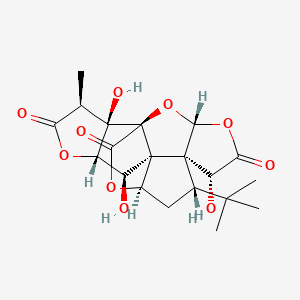 |
||||
| Formula |
C20H24O10
|
||||
| IUPAC Name |
(1R,3R,6R,7S,8S,10R,11R,12R,13S,16S,17R)-8-tert-butyl-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclo[8.7.2.01,11.03,7.07,11.013,17]nonadecane-5,15,18-trione
|
||||
| Canonical SMILES |
CC1C(=O)OC2C1(C34C(=O)OC5C3(C2O)C6(C(C5)C(C)(C)C)C(C(=O)OC6O4)O)O
|
||||
| InChI |
InChI=1S/C20H24O10/c1-6-12(23)28-11-9(21)18-8-5-7(16(2,3)4)17(18)10(22)13(24)29-15(17)30-20(18,14(25)27-8)19(6,11)26/h6-11,15,21-22,26H,5H2,1-4H3/t6-,7+,8-,9+,10+,11+,15+,17+,18+,19-,20-/m1/s1
|
||||
| InChIKey |
SQOJOAFXDQDRGF-MMQTXUMRSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
Male 6-month-old senescence-resistant R1 (SAMR1) and SAMP8 mice (weight, 28-35 g) were purchased from Beijing SPF Biotechnology. First, mice were placed in the center of an empty testing arena (40 x 40 x 40 cm) and allowed to move freely for adaptation. Next, in the training stage, two similar objects were presented in the testing arena and mice were allowed to explore for 10 min, for 3 consecutive days. On day 4, one of the two familiar objects was replaced by a new object. The time of exploring a novel object or familiar object in 10 min was recorded.
Click to Show/Hide
|
||||
| Response regulation | Ginkgolide B attenuated Alzheimer's disease (AD)-related cognitive impairment through the regulation of oxidative stress, neuroinflammation and ferroptosis, and that GB-induced protection in AD is dependent on the inhibition of ferroptosis. Furthermore, the involvement of Nrf2/GPX4 pathway-regulated ferroptosis in the GB-related protective effects on the AD mouse model. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
Male 6-month-old senescence-resistant R1 (SAMR1) and SAMP8 mice (weight, 28-35 g) were purchased from Beijing SPF Biotechnology. First, mice were placed in the center of an empty testing arena (40 x 40 x 40 cm) and allowed to move freely for adaptation. Next, in the training stage, two similar objects were presented in the testing arena and mice were allowed to explore for 10 min, for 3 consecutive days. On day 4, one of the two familiar objects was replaced by a new object. The time of exploring a novel object or familiar object in 10 min was recorded.
Click to Show/Hide
|
||||
| Response regulation | Ginkgolide B attenuated Alzheimer's disease (AD)-related cognitive impairment through the regulation of oxidative stress, neuroinflammation and ferroptosis, and that GB-induced protection in AD is dependent on the inhibition of ferroptosis. Furthermore, the involvement of Nrf2/GPX4 pathway-regulated ferroptosis in the GB-related protective effects on the AD mouse model. | ||||
