Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0097)
| Name |
Entacapone
|
||||
|---|---|---|---|---|---|
| Synonyms |
ENTACAPONE; 130929-57-6; Comtan; Comtess; Entacaponum; OR-611; Entacapona; Entacaponum [INN-Latin]; Entacapona [INN-Spanish]; (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylacrylamide; 116314-67-1; Entacapone teva; Entacapone orion; (E)-alpha-Cyano-N,N-diethyl-3,4-dihydroxy-5-nitrocinnamamide; 2-Cyano-N,N-diethyl-3-(3,4-dihydroxy-5-nitrophenyl)propenamide; (2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enamide; (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enamide; CHEMBL953; 2-Propenamide, 2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-; (E)-2-cyano-3-(3,4-dihydroxy-5-nitro-phenyl)-N,N-diethyl-prop-2-enamide; CHEBI:4798; DTXSID5046439; N,N-diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl) acrylamide; 4975G9NM6T; (2E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide; NCGC00164555-01; Entacom; 2-Propenamide, 2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-, (2E)-; Entacapone [USAN:INN]; DTXCID3026439; OR 611; (E)-Entacapone; (~{E})-2-cyano-~{N},~{N}-diethyl-3-[3-nitro-4,5-bis(oxidanyl)phenyl]prop-2-enamide; Comtan (TN); CAS-130929-57-6; SR-05000001452; UNII-4975G9NM6T; COM-998; Entacapone [USAN:USP:INN:BAN]; ENTACAPONE [MI]; ENTACAPONE [INN]; ENTACAPONE [JAN]; ENTACAPONE [USAN]; (E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide; ENTACAPONE [VANDF]; ENTACAPONE [MART.]; ENTACAPONE [USP-RS]; ENTACAPONE [WHO-DD]; SCHEMBL34504; SCHEMBL34505; BIDD:GT0026; ENTACAPONE [EMA EPAR]; 2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-n,n-diethyl-2-propenamide; Entacapone (JP17/USP/INN); GTPL6647; ENTACAPONE [ORANGE BOOK]; SCHEMBL13596593; HSDB 8251; ENTACAPONE [EP MONOGRAPH]; JRURYQJSLYLRLN-BJMVGYQFSA-N; ENTACAPONE [USP MONOGRAPH]; HMS2089O16; HMS3713B20; HMS3885K09; OR611; STALEVO COMPONENT ENTACAPONE; CORBILTA COMPONENT ENTACAPONE; EX-A1130; Tox21_112184; AC-393; BDBM50108879; MFCD00866580; s3147; AKOS015907685; AKOS015965009; ENTACAPONE COMPONENT OF STALEVO; Tox21_112184_1; BCP9000645; CCG-213064; CS-1266; DB00494; ENTACAPONE COMPONENT OF CORBILTA; NCGC00164555-02; NCGC00164555-03; NCGC00164555-10; HY-14280; E0961; SW199035-2; C07943; D00781; AB01275450-01; AB01275450_02; AB01275450_03; A806167; A922031; Q416444; J-005902; J-008069; SR-05000001452-1; SR-05000001452-2; SR-05000001452-3; BRD-K83636919-001-01-4; LEVODOPA/CARBIDOPA/ENTACAPONE ORION COMPONENT ENTACAPONE; (E)-N,N-diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)acrylamide; ENTACAPONE COMPONENT OF LEVODOPA/CARBIDOPA/ENTACAPONE ORION; (E)-.ALPHA.-CYANO-N,N-DIETHYL-3,4-DIHYDROXY-5-NITROCINNAMAMIDE; (E)-N, N-diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)acrylamide; (E)-2-cyano-N,N-diethyl-3-[3-nitro-4,5-bis(oxidanyl)phenyl]prop-2-enamide; 2-Propenamide,2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-,(2E)-; PD9
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
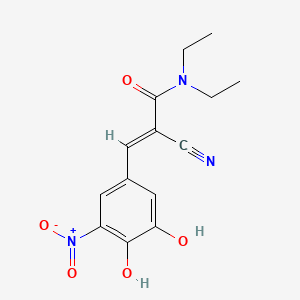 |
||||
| Formula |
C14H15N3O5
|
||||
| IUPAC Name |
(E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enamide
|
||||
| Canonical SMILES |
CCN(CC)C(=O)C(=CC1=CC(=C(C(=C1)O)O)[N+](=O)[O-])C#N
|
||||
| InChI |
InChI=1S/C14H15N3O5/c1-3-16(4-2)14(20)10(8-15)5-9-6-11(17(21)22)13(19)12(18)7-9/h5-7,18-19H,3-4H2,1-2H3/b10-5+
|
||||
| InChIKey |
JRURYQJSLYLRLN-BJMVGYQFSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Acute kidney failure | ICD-11: GB60 | |||
| Responsed Regulator | Sequestosome-1 (SQSTM1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks; 20-25 g) were purchased from LINGCHANG BIOTECH (China). Mice were divided into four groups: (i) sham, (ii) I/R, (iii) I/R+entacapone, and (iv) I/R + Fer-1. Entacapone (15 mg/kg bodyweight) was dissolved in sodium carboxymethyl cellulose (0.5%) and administered (i.g.) to mice. Mice in the sham group were administered (i.g.) an equal volume of solvent. Fer-1 was dissolved in 5% dimethyl sulfoxide + 30% polyethylene glycol-400 + 60% saline and injected (i.p.). Mice were treated three times per day for 3 days in advance. Before I/R, mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.). The abdomen was exposed and bilateral renal pedicles were clamped to induce renal I/R. After 25 min, the arterial clamps were removed. A body temperature of 37 was maintained throughout the procedure. The sham group underwent the same procedure except for clamping of the renal pedicle. Mice were killed 24 h after reperfusion, and kidney and blood samples were collected for experimentation.
Click to Show/Hide
|
||||
| Response regulation | Entacapone upregulates p62 (SQSTM1) expression and affects the p62-KEAP1-NRF2 pathway, thereby upregulating nuclear translocation of NRF2. This action results in increased expression of the downstream SLC7A11, and significant suppression of oxidative stress and ferroptosis. Entacapone may serve as a novel strategy to improve treatment of, and recovery from, ischemia/reperfusion-induced acute kidney injury (I/R-AKI). | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Acute kidney failure | ICD-11: GB60 | |||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks; 20-25 g) were purchased from LINGCHANG BIOTECH (China). Mice were divided into four groups: (i) sham, (ii) I/R, (iii) I/R+entacapone, and (iv) I/R + Fer-1. Entacapone (15 mg/kg bodyweight) was dissolved in sodium carboxymethyl cellulose (0.5%) and administered (i.g.) to mice. Mice in the sham group were administered (i.g.) an equal volume of solvent. Fer-1 was dissolved in 5% dimethyl sulfoxide + 30% polyethylene glycol-400 + 60% saline and injected (i.p.). Mice were treated three times per day for 3 days in advance. Before I/R, mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.). The abdomen was exposed and bilateral renal pedicles were clamped to induce renal I/R. After 25 min, the arterial clamps were removed. A body temperature of 37 was maintained throughout the procedure. The sham group underwent the same procedure except for clamping of the renal pedicle. Mice were killed 24 h after reperfusion, and kidney and blood samples were collected for experimentation.
Click to Show/Hide
|
||||
| Response regulation | Entacapone upregulates p62 (SQSTM1) expression and affects the p62- KEAP1-NRF2 pathway, thereby upregulating nuclear translocation of NRF2. This action results in increased expression of the downstream SLC7A11, and significant suppression of oxidative stress and ferroptosis. Entacapone may serve as a novel strategy to improve treatment of, and recovery from, ischemia/reperfusion-induced acute kidney injury (I/R-AKI). | ||||
