Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0025)
| Name |
Glycyrrhizin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Glycyrrhizic acid; glycyrrhizin; 1405-86-3; Glycyrrhizinic acid; Glycyron; Glycyrrhetinic acid glycoside; potenlini; Glizigen; glycyrrhizate; glyzyrrhizin; 18-beta-Glycyrrhizic acid; beta-Glycyrrhizin; 18beta-Glycyrrhizic acid; Glycyrrizin; Rizinsan K2 A2 (free acid); HSDB 496; CHEBI:15939; EINECS 215-785-7; Glycyrrhizin [JAN]; .beta.-glycyrrhizin; NSC 167409; NSC 234419; NSC-167409; NSC-234419; UNII-6FO62043WK; BRN 0077922; CCRIS 8444; DTXSID8047006; 6FO62043WK; C42H62O16; 18.beta.-glycyrrhizic acid; Glycyrrhizinate; CHEMBL441687; NSC 2800; DTXCID6027006; 4-18-00-05156 (Beilstein Handbook Reference); (3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en-3-yl-2-O-beta-D-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid; 20beta-Carboxy-11-oxo-30-norolean-12-en-3beta-yl-2-O-beta-D-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid; 30-hydroxy-11,30-dioxoolean-12-en-3beta-yl (2-O-beta-D-glucopyranosyluronic acid)-alpha-D-glucopyranosiduronic acid; alpha-D-Glucopyranosiduronic acid, (3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-beta-D-glucopyranuronosyl-; alpha-D-Glucopyranosiduronic acid, (3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en-3-yl-2-O-beta-D-glucopyranuron osyl-; GLYCYRRHIZIN (II); GLYCYRRHIZIN [II]; Glycyrrhitin; Dermacrin; (3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-beta-D-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid; GLYCYRRHIZIC ACID (MART.); GLYCYRRHIZIC ACID [MART.]; GLYCYRRHIZIC ACID (USP-RS); GLYCYRRHIZIC ACID [USP-RS]; NSC167409; Glycyram; Neo-Umor; Acid, Glycyrrhizic; (2S,3S,4S,5R,6S)-6-{[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl]oxy}-5-{[(2R,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxyoxan-2-yl]oxy}-3,4-dihydroxyoxane-2-carboxylic acid; CAS-1405-86-3; Acid, Glycyrrhizinic; MFCD00065194; NSC234419; NCGC00183128-01; (2S,3S,4S,5R,6R)-6-[(2S,3R,4S,5S,6S)-2-[[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-2,3,4a,5,6,7,8,9,10,12,12a,14a-dodecahydro-1H-picen-3-yl]oxy]-6-carboxy-4,5-dihydroxy-tetrahydropyran-3-yl]oxy-3,4,5-trihydroxy-tetrahydropyran-2-carboxylic acid; ammonium-glycyrrhizinate; Glycyrrhizic acid, 2K; beta-Glycyrrhizinic acid; GLYCYRRHIZIN [HSDB]; SCHEMBL17684; alpha-D-glucopyranosiduronic acid, (3 beta,20 beta)-20-carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-beta-D-glucopyranuronosyl-; BIDD:ER0363; GLYCYRRHIZIC ACID [MI]; GTPL4688; GLYCYRRHIZIC ACID [INCI]; Glycyrrhizin (Glycyrrhizic Acid); LPLVUJXQOOQHMX-QWBHMCJMSA-N; GLYCYRRHIZIC ACID [WHO-DD]; HY-N0184; Tox21_111520; Tox21_113426; Tox21_303493; BDBM50185127; HMDB:0029843; s2302; AKOS015893086; AKOS015969345; CCG-270511; CS-7695; DB13751; GM-1292; NCGC00257455-01; NCGC00386162-01; NCGC00386162-02; (3beta,20beta)-20-Carboxy-11-oxo-30-norlean-12-en-3-yl-2-O-beta-1- 7-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid; alpha-D-Glucopyranosiduronic acid, (3beta,20beta)-20-carboxy-11-oxo-30-norlean-12-en-3-yl-2-O-beta-1- 7-glucopyranuronosyl-; AS-13001; E958; G0150; AB01566834_01; EN300-25023649; Q418705; Q-201172; BRD-K83486494-318-01-5; GLYCYRRHIZIC ACID (GLYCYRRHIZIN) (CONSTITUENT OF LICORICE); GLYCYRRHIZIC ACID (GLYCYRRHIZIN) (CONSTITUENT OF LICORICE) [DSC]; Glycyrrhizic acid, United States Pharmacopeia (USP) Reference Standard; Glycyrrhizic Acid, Pharmaceutical Secondary Standard; Certified Reference Material; (2S,3S,4S,5R,6R)-6-[(2S,3R,4S,5S,6S)-2-[[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-2,3,4a,5,6,7,8,9,10,12,12a,14a-dodecahydro-1H-picen-3-yl]oxy]-6-carboxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid; (2S,3S,4S,5R,6R)-6-[(2S,3R,4S,5S,6S)-2-[[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-2,3,4a,5,6,7,8,9,10,12,12a,14a-dodecahydro-1H-picen-3-yl]oxy]-6-carboxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylicacid; (3.beta.,20.beta.)-20-Carboxy-11-oxo-30-norlean-12-en-3-yl-2-O-.beta.-1- 7-glucopyranuronosyl-.alpha.-D-glucopyranosiduronic acid; (3.BETA.,20.BETA.)-20-CARBOXY-11-OXO-30-NOROLEAN-12-EN-3-YL 2-O-.BETA.-D-GLUCOPYRANURONOSYL-.ALPHA.-D-GLUCOPYRANOSIDURONIC ACID; (3beta,20beta)-20-Carboxy-11-oxo-30-norlean-12-en-3-yl-2-O-beta-1-7-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid; 20BETA-CARBOXY-11-OXO-30-NOROLEAN-12-EN-3BETA-YL-2-O-BE TA-D-GLUCOPYRANURONOSYL-ALPHA-D-GLUCOPYRANOSIDURONIC ACID; alpha-D-Glucopyranosiduronic acid, (3beta,20beta)-20-carboxy-11-oxo-30-norlean-12-en-3-yl-2-O-beta-1-7-glucopyranuronosyl-
Click to Show/Hide
|
||||
| Status |
Phase 3
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
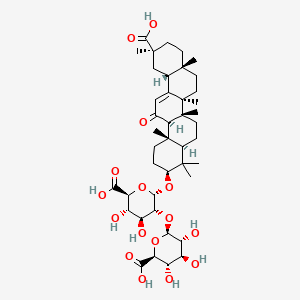 |
||||
|
3D MOL
|
|||||
| Formula |
C42H62O16
|
||||
| IUPAC Name |
(2S,3S,4S,5R,6R)-6-[(2S,3R,4S,5S,6S)-2-[[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-2,3,4a,5,6,7,8,9,10,12,12a,14a-dodecahydro-1H-picen-3-yl]oxy]-6-carboxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
|
||||
| Canonical SMILES |
CC1(C2CCC3(C(C2(CCC1OC4C(C(C(C(O4)C(=O)O)O)O)OC5C(C(C(C(O5)C(=O)O)O)O)O)C)C(=O)C=C6C3(CCC7(C6CC(CC7)(C)C(=O)O)C)C)C)C
|
||||
| InChI |
InChI=1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-30(26(47)25(46)29(57-35)33(51)52)58-34-27(48)23(44)24(45)28(56-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25-,26-,27+,28-,29-,30+,31+,34-,35-,38+,39-,40-,41+,42+/m0/s1
|
||||
| InChIKey |
LPLVUJXQOOQHMX-QWBHMCJMSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hypoxic ischemic brain injury | ICD-11: 8B24 | |||
| Responsed Regulator | High mobility group protein B1 (HMGB1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPCNs (Rat primary cortical neurons) | ||||
| In Vivo Model |
Male and female neonatal SpragueDawley rats on postpartum day 7 (P7) were provided by SPF Biotechnology (Beijing, China). Each animal was anesthetized with isoflurane (4% for induction, 2% for maintenance), the skin was incised, and the left common carotid artery was exposed. This artery was ligated with a 5-0 suture and cut, and the skin was sutured closed. Next, the pups recovered for 1 h with their mother. Subsequently, the pups were placed in a hypoxia chamber (8% O2 + 92% N2 mixture) for 2 h. After 2 h of hypoxia, the animals were placed back with their dam.
Click to Show/Hide
|
||||
| Response regulation | Glycyrrhizin (GL) not only inhibited ferroptosis induced by RSL3 and oxygen-glucose deprivation in vitro but also inhibited ferroptosis induced by hypoxic-ischemic brain damage (HIBD) in vivo. GL could suppress the occurrence of neuronal ferroptosis and reduce neuronal loss in HIBD via the HMGB1/GPX4 pathway. | ||||
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Hepatoblastoma | ICD-11: DB91 | |||
| Responsed Regulator | High mobility group protein B1 (HMGB1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
In total, 40 male specific- pathogen-free C57BL/6 mice (Hubei Animal Experimental Center) 6-8 weeks old. The mice were randomly divided into 5 groups: The normal group, model group, 15 mg/kg GLY group, 30 mg/kg GLY group and 60 mg/kg GLY group. Except for the normal group, the other four groups of mice were injected intraperitoneally with D-GalN (400 mg/kg) and LPS (100 ug/kg) to induce the ALF model. According to a previous study on GLY gavage doses, three doses of GLY (15, 30 and 60 mg/kg/day) intervention groups were used. A total of 24 mice were divided into three groups. Mice received gavage with different doses of GLY for 3 days before induction of the ALF model.
Click to Show/Hide
|
||||
| Response regulation | The HMGB1 inhibitor glycyrrhizin (GLY) significantly reduced the degree of ferroptosis during acute liver failure (ALF) by inhibiting oxidative stress. Treatment with GLY reduced the degree of liver damage, the expression of HMGB1 was decreased, and the levels of Nrf2, HO1 and GPX4 were increased. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Hepatoblastoma | ICD-11: DB91 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
In total, 40 male specific- pathogen-free C57BL/6 mice (Hubei Animal Experimental Center) 6-8 weeks old. The mice were randomly divided into 5 groups: The normal group, model group, 15 mg/kg GLY group, 30 mg/kg GLY group and 60 mg/kg GLY group. Except for the normal group, the other four groups of mice were injected intraperitoneally with D-GalN (400 mg/kg) and LPS (100 ug/kg) to induce the ALF model. According to a previous study on GLY gavage doses, three doses of GLY (15, 30 and 60 mg/kg/day) intervention groups were used. A total of 24 mice were divided into three groups. Mice received gavage with different doses of GLY for 3 days before induction of the ALF model.
Click to Show/Hide
|
||||
| Response regulation | The HMGB1 inhibitor glycyrrhizin (GLY) significantly reduced the degree of ferroptosis during acute liver failure (ALF) by inhibiting oxidative stress. Treatment with GLY reduced the degree of liver damage, the expression of HMGB1 was decreased, and the levels of Nrf2, HO1 and GPX4 were increased. | ||||
References
