Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0179)
| Name |
Ibrutinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ibrutinib; 936563-96-1; PCI-32765; IMBRUVICA; PCI 32765; Ibrutinib (PCI-32765); PCI-32765 (Ibrutinib); CRA-032765; (R)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one; Pc-32765; 1X70OSD4VX; PCI-32765-00; 1-[(3R)-3-[4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one; CHEBI:76612; PCI32765; 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one; 1-{(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl}prop-2-en-1-one; 2-Propen-1-one, 1-((3R)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidin-1-yl)-1-piperidinyl)-; C25H24N6O2; (R)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one.; 1-((3R)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidin-1-yl)piperidin-1- yl)prop-2-en-1-one; Ibrutinib [USAN]; Ibrutinib [USAN:INN]; UNII-1X70OSD4VX; ibrutinibum; JNJ 02; Imbruvica (TN); Ibrutinib- Bio-X; 1-{(3R)-3-[4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1- yl}prop-2-en-1-one; 2-Propen-1-one, 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-; CRA 032765; Ibrutinib, Free Base; 1-[(3R)-3-[4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one; IBRUTINIB [INN]; IBRUTINIB [JAN]; IBRUTINIB [MI]; Ibrutinib (JAN/USAN); IBRUTINIB [VANDF]; IBRUTINIB [WHO-DD]; Imbruvica; PCI-32765; MLS006010041; SCHEMBL201859; GTPL6912; IBRUTINIB [ORANGE BOOK]; CHEMBL1873475; HSDB 8260; DTXSID60893450; EX-A066; XYFPWWZEPKGCCK-GOSISDBHSA-N; AMY27873; BDBM50357312; MFCD20261150; NSC800769; AKOS022185476; DB09053; EX-5960; NSC-800769; NCGC00187912-01; NCGC00187912-02; NCGC00187912-03; NCGC00187912-12; (R)-1-(3-(4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo-[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one; AC-26942; BI164531; HY-10997; SMR004701213; SW218096-2; EN300-97039; D10223; A1-01649; J-523872; Q5984881; Z1302446275; (R)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-ylprop-2-en-1-one; 1-((R)-3-(4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one; 1-[(3R)-3-[4-Amino-3-(4-phenoxyphenyl)pyrazolo[3, 4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one
Click to Show/Hide
|
||||
| Structure |
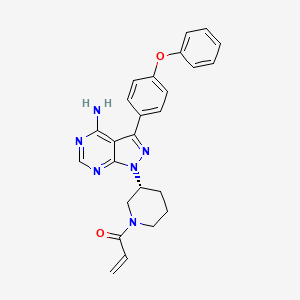 |
||||
| Formula |
C25H24N6O2
|
||||
| IUPAC Name |
1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one
|
||||
| Canonical SMILES |
C=CC(=O)N1CCCC(C1)N2C3=NC=NC(=C3C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)N
|
||||
| InChI |
InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1
|
||||
| InChIKey |
XYFPWWZEPKGCCK-GOSISDBHSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Regulator | Tyrosine-protein kinase BTK (BTK) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| LS513 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1386 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| SW1116 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0544 | ||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| HT-29 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| In Vivo Model |
Sixty mice were randomly divided into six groups, (1) the CRC model group (model), (2) mice with RSL3 treatment, (3) mice with Erastin treatment, (4) mice with Ibrutinib treatment, (5) mice with RSL3 and Ibrutinib treatment, and (6) Erastin and Ibrutinib group. Murine subcutaneous tumor model and xenograft tumor mouse model were established and please refer to supplemental method for details. For CRC model group, the mice were treated with PBS for two weeks. For RSL3 group, the mice were intraperitoneal injected with RSL3 (5 mg/kg daily) for two weeks. For Erastin group, the mice were intraperitoneal injected with Erastin (30 mg/kg, twice every other day) for two weeks. For Ibrutinib treatment group, mice were administered in drinking water at a concentration of 0.16 mg/ml for two weeks. Mice were also treated in combination with RSL and Ibrutinib or Erastin and Ibrutinib.
Click to Show/Hide
|
||||
| Response regulation | Ibrutinib inhibited BTK, which prevented Nrf2 translocating to cell nucleus and the activation of the Nrf2 dependent antioxidant genes during oxidative stress conditions and eventually enhanced the sensitivity of Colorectal cancer (CRC) cells to ferroptosis. | ||||
