Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0061)
| Name |
Hesperidin
|
||||
|---|---|---|---|---|---|
| Synonyms |
hesperidin; Cirantin; 520-26-3; Hesperidoside; Hesper bitabs; Hesperetin 7-rhamnoglucoside; Hesperetin 7-rutinoside; Hesperetin-rutinosid; Hesperidine; Ciratin; (S)-(-)-Hesperidin; Hesperetin 7-O-rutinoside; NSC 44184; CCRIS 3940; EINECS 208-288-1; (2S)-Hesperidin; UNII-E750O06Y6O; BRN 0075140; DIOSVEIN; Hesperetin-7-O-rhamnoglucoside; Hesperidin, (2S)-; DTXSID9044328; Hesperetin-7-rutinoside; CHEBI:28775; E750O06Y6O; USAF CF-3; Hesperitin-7-rhamnoglucoside; Hesperidin, (S)-(-)-; 3',5'-Dihydroxy-4'-methoxy-7-rutinosyloxyflavan-4-on; MLS001304066; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one; DTXCID7024328; EC 208-288-1; 5-18-05-00218 (Beilstein Handbook Reference); Hesperidin (JAN); Hesperetin, 7-(6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside); C28H34O15; NSC-44184; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-7-yl 6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-3,4-dihydro-2H-1-benzopyran-4-one; (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)chroman-4-one; 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydo-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (S)-; 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((6-O-alpha-L-rhamnopyranosyl-beta-D-glucopyranosyl)oxy)-4-chromanon; 7-((6-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one, (S)-; 7-(6-O-Desoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyloxy)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-chromanon; SMR000718775; HESPERIDIN [JAN]; MFCD00075663; HESPERIDIN (MART.); HESPERIDIN [MART.]; HESPERIDIN (USP-RS); HESPERIDIN [USP-RS]; (s)-7-[[6-o-(6-deoxy-alpha-l-mannopyranosyl)-beta-d-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one; Aurantiamarin (Methyl Hesperidin); 3',5'-DIHYDROXY-4'-METHOXY-7-RUTINOSYLOXYFLAVAN-4-ONE; hesperetin 7-(6-O-alpha-L-rhamnopyranosyl)-beta-D-glucopyranoside; NSC44184; Hesperidin 2S; 2S, Hesperidin; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxy-phenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl]oxymethyl]tetrahydropyran-2-yl]oxy-chroman-4-one; Hesperitin-7-rutinoside; Hesperetin 7 Rutinoside; SR-01000799145; Hesperetin 7 Rhamnoglucoside; Hesperidina; 7-Rhamnoglucoside, Hesperetin; Hesperidin,(S); NCGC00016481-01; (2S)-7-[[6-O-(6-Deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one (Hesperidin); (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-2H-pyran-2-yloxy)chroman-4-one; CAS-520-26-3; Hesperetin Glycoside; Hesperidin, >=80%; HESPERIDIN [MI]; DIOSMIN [NDI]; Prestwick3_000400; HESPERIDIN [INCI]; HESPERIDIN [VANDF]; Glucopyranoside, hesperetin-7 6-O-(6-deoxy-alpha-L-mannopyranosyl)-, beta-D-; HESPERIDIN [WHO-DD]; SCHEMBL94586; BSPBio_000619; 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (S)-; cid_10621; Hesperidin, analytical standard; BPBio1_000681; CHEMBL449317; NDI 590 [FDMS]; BCBcMAP01_000136; BDBM61776; NDI 590; 2H-pyran-2-yloxy)chroman-4-one; QUQPHWDTPGMPEX-QJBIFVCTSA-N; HMS2096O21; HMS2233I03; HMS3713O21; Tox21_110448; s2309; AKOS015895450; CCG-208580; CS-5631; DB04703; KS-5308; SMP1_000149; NCGC00179501-01; 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl)oxy)-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2S)-; HY-15337; AB00513829; H0049; C09755; D01038; (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-; EN300-7419024; Q385937; Hesperidin, primary pharmaceutical reference standard; methyltetrahydro-2H-pyran-2-yloxy)methyl)tetrahydro-; SR-01000799145-4; SR-01000799145-5; SR-01000799145-7; 7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-; BRD-K38903228-001-02-8; BRD-K38903228-001-13-5; Hesperidin, European Pharmacopoeia (EP) Reference Standard; Hesperidin, Pharmaceutical Secondary Standard; Certified Reference Material; Flavanone, 3',5,7-trihydroxy-4'-methoxy-, 7-(6-O-alpha-L-rhamnosyl-D-glucoside); (2S)-2-(4-methoxy-3-oxidanyl-phenyl)-7-[(2S,3R,4S,5S,6R)-6-[[(2R,3R,4R,5R,6S)-6-methyl-3,4,5-tris(oxidanyl)oxan-2-yl]oxymethyl]-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5-oxidanyl-2,3-dihydrochromen-4-one; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-2-oxanyl]oxymethyl]-2-oxanyl]oxy]-3,4-dihydro-2H-1-benzopyran-4-one; (2S)-7-((6-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-2,3-DIHYDRO-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE; (2S)-7-((6-O-(6-DEOXY-alpha-L-MANNOPYRANOSYL)-beta-D-GLUCOPYRANOSYL)OXY)-2,3-DIHYDRO-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-4H-1-BENZOPYRAN-4-ONE; (S)-7-[[6-O-(6-Deoxy-.alpha.-L-mannopyranosyl)-.beta.-D-glucopyranosyl]oxy]-2,3-dihydro-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one; 4H-1-BENZOPYRAN-4-ONE, 7-((6-O-(6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)-.BETA.-D-GLUCOPYRANOSYL)OXY)-2,3-DIHYDO-5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-, (S)-; 5-HYDROXY-2-(3-HYDROXY-4-METHOXYPHENYL)-7-((6-O-.ALPHA.-L-RHAMNOPYRANOSYL-.BETA.-D-GLUCOPYRANOSYL)OXY)-4-CHROMANON
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
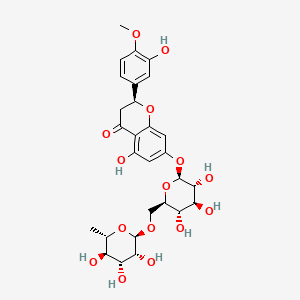 |
||||
| Formula |
C28H34O15
|
||||
| IUPAC Name |
(2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one
|
||||
| Canonical SMILES |
CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC(=C(C=C5)OC)O)O)O)O)O)O)O)O
|
||||
| InChI |
InChI=1S/C28H34O15/c1-10-21(32)23(34)25(36)27(40-10)39-9-19-22(33)24(35)26(37)28(43-19)41-12-6-14(30)20-15(31)8-17(42-18(20)7-12)11-3-4-16(38-2)13(29)5-11/h3-7,10,17,19,21-30,32-37H,8-9H2,1-2H3/t10-,17-,19+,21-,22+,23+,24-,25+,26+,27+,28+/m0/s1
|
||||
| InChIKey |
QUQPHWDTPGMPEX-QJBIFVCTSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Intervertebral disc degeneration | ICD-11: FA80 | |||
| Responsed Regulator | Transcription factor p65 (RELA) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hNPCs (Human nucleus pulposus cells) | ||||
| In Vivo Model |
Male C57BL/6 mice (10-12 weeks) were devoted to generate needle puncture-induced intervertebral disc degeneration model. For intervertebral disc degeneration (IVDD) treatment, hesperidin was administratedorally and the dose was calculated in reference to previous studies using themetrological conversion formula between human and mouse. The dosein mice is = 5.5 mg/kg x 70 kg x 0.0026/20g = 9.1 x 5.5 mg/kg = 50.05 mg/kg ~50 mg/kg.
Click to Show/Hide
|
||||
| Response regulation | The current study proved for the first time that Hesperidin may protect HNP cells from degeneration by suppressing ferroptosis in an oxidative stress-dependent via enhancing the expression of Nrf2 and suppressing the NF-kB pathway. The evidence will provide a possible basis for future targeted treatment for intervertebral disc degeneration. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Intervertebral disc degeneration | ICD-11: FA80 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hNPCs (Human nucleus pulposus cells) | ||||
| In Vivo Model |
Male C57BL/6 mice (10-12 weeks) were devoted to generate needle puncture-induced intervertebral disc degeneration model. For intervertebral disc degeneration (IVDD) treatment, hesperidin was administratedorally and the dose was calculated in reference to previous studies using themetrological conversion formula between human and mouse. The dosein mice is = 5.5 mg/kg x 70 kg x 0.0026/20g = 9.1 x 5.5 mg/kg = 50.05 mg/kg ~50 mg/kg.
Click to Show/Hide
|
||||
| Response regulation | Hesperidin may protect HNP cells from degeneration by suppressing ferroptosis in an oxidative stress-dependent via enhancing the expression of Nrf2 and suppressing the NF-B pathway. The evidence will provide a possible basis for future targeted treatment for intervertebral disc degeneration. | ||||
