Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0217)
| Name |
Withaferin A
|
||||
|---|---|---|---|---|---|
| Synonyms |
Withaferin A; 5119-48-2; Withaferine A; NSC 273757; WITHAFERIN DERIV JPR, IOWA U. COMPOUND; CHEBI:69120; NSC-101088; NSC101088; L6DO3QW4K5; CHEMBL517080; Ashwagandha; NSC-273757; 4beta,27-dihydroxy-1-oxo-5beta,6beta-epoxywitha-2,24-dienolide; (1S,2R,6S,7R,9R,11S,12S,15R,16S)-6-hydroxy-15-[(1S)-1-[(2R)-5-(hydroxymethyl)-4-methyl-6-oxo-2,3-dihydropyran-2-yl]ethyl]-2,16-dimethyl-8-oxapentacyclo[9.7.0.02,7.07,9.012,16]octadec-4-en-3-one; (6S,7R,9R)-6-Hydroxy-15-[(2R,3R)-3-hydroxy-4-(4-methyl-5-oxo-2H-furan-3-yl)butan-2-yl]-2,16-dimethyl-8-oxapentacyclo[9.7.0.02,7.07,9.012,16]octadec-4-en-3-one; UNII-L6DO3QW4K5; BRN 1335150; 5.beta.-Ergosta-2, 5,6.beta.-epoxy-4.beta.,22,27-trihydroxy-1-oxo-, .delta.-lactone, (20S,22R)-; Ergosta-2, 5,6-epoxy-4,22,27-trihydroxy-1-oxo-, .delta.-lactone, (4.beta.,5.beta.,6.beta.,22R)-; NSC 101088; WITHAFERIN A [MI]; 5-19-06-00604 (Beilstein Handbook Reference); MLS006010687; SCHEMBL157208; Withaferin A, analytical standard; Withaferin A, >=95% (HPLC); HY-N2065; BDBM50599323; MFCD10687098; NSC273757; s8587; AKOS040758787; (4beta,5beta,6beta,22R)-4,27-dihydroxy-5,6:22,26-diepoxyergosta-2,24-diene-1,26-dione; NCGC00180796-02; (4beta,5beta,6beta,22R)-5,6-Epoxy-4,22,27-trihydroxy-1-oxoergosta-2,24-dien-26-oic acid, delta-lactone; 5-beta-Ergosta-2,24-dien-26-oic acid, 5,6-beta-epoxy-4-beta,22,27-trihydroxy-1-oxo-, delta-lactone, (20S,22R)-; 5beta-Ergosta-2,24-dien-26-oic acid, 5,6beta-epoxy-4beta,22,27-trihydroxy-1-oxo-, delta-lactone, (20S,22R)-; AS-77575; Ergosta-2,24-dien-26-oic acid, 5,6-epoxy-4,22,27-trihydroxy-1-oxo-, gamma-lactone, (4bta,5beta,6beta,22R)-; NCI60_000031; SMR004701668; CS-0018562; Withaferin A 100 microg/mL in Acetonitrile; C08841; A871350; A1-06845; Q6606395; BRD-K88378636-001-01-0; WLN: T3 F5 E666 1A R AXO OV PU CH&TTTTJ J1 N1 RQ IY1&- FT6OV CUTJ C1Q D1; (4.BETA.,5.BETA.,6.BETA.,22R)-4,27-DIHYDROXY-5,6:22,26-DIEPOXYERGOSTA-2,24-DIENE-1,26-DIONE; (4S,4aR,5aR,6aS,6bS,9R,9aS,11aS,11bR)-4-hydroxy-9-((S)-1-((R)-5-(hydroxymethyl)-4-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-9a,11b-dimethyl-5a,6,6a,6b,7,8,9,9a,10,11,11a,11b-dodecahydrocyclopenta[1,2]phenanthro[8a,9-b]oxiren-1(4H)-one; Ergosta-2,24-dien-26-oicacid, 5,6-epoxy-4,22,27-trihydroxy-1-oxo-, d-lactone, (4b,5b,6b,22R)-; NCGC00180796-02_C28H38O6_(4beta,5beta,6beta,22R)-4,27-Dihydroxy-5,6:22,26-diepoxyergosta-2,24-diene-1,26-dione
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
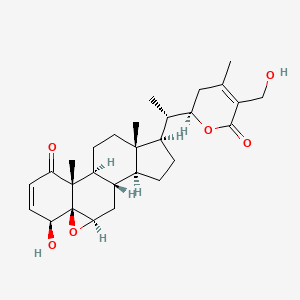 |
||||
| Formula |
C28H38O6
|
||||
| IUPAC Name |
(1S,2R,6S,7R,9R,11S,12S,15R,16S)-6-hydroxy-15-[(1S)-1-[(2R)-5-(hydroxymethyl)-4-methyl-6-oxo-2,3-dihydropyran-2-yl]ethyl]-2,16-dimethyl-8-oxapentacyclo[9.7.0.02,7.07,9.012,16]octadec-4-en-3-one
|
||||
| Canonical SMILES |
CC1=C(C(=O)OC(C1)C(C)C2CCC3C2(CCC4C3CC5C6(C4(C(=O)C=CC6O)C)O5)C)CO
|
||||
| InChI |
InChI=1S/C28H38O6/c1-14-11-21(33-25(32)17(14)13-29)15(2)18-5-6-19-16-12-24-28(34-24)23(31)8-7-22(30)27(28,4)20(16)9-10-26(18,19)3/h7-8,15-16,18-21,23-24,29,31H,5-6,9-13H2,1-4H3/t15-,16-,18+,19-,20-,21+,23-,24+,26+,27-,28+/m0/s1
|
||||
| InChIKey |
DBRXOUCRJQVYJQ-CKNDUULBSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | ||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | ||
| Pathway Response | Pathways in cancer | hsa05200 | ||
| Ferroptosis | hsa04216 | |||
| Cell adhesion molecules | hsa04514 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell invasion | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | |
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| Response regulation | Withaferin A may attenuate the metastatic potential and sorafenib resistance by regulating Keap1/Nrf2-associated EMT and ferroptosis. Thus, Withaferin A may serve as a promising agent for Hepatocellular carcinoma therapy, especially for advanced hepatocellular carcinoma. | |||
