Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0111)
| Name |
Naringenin
|
||||
|---|---|---|---|---|---|
| Synonyms |
naringenin; 480-41-1; Salipurol; (S)-Naringenin; (S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one; pelargidanon; naringetol; (2S)-Naringenin; salipurpol; Naringenine; Asahina; NARIGENIN; NSC 11855; CCRIS 5839; (-)-(2S)-Naringenin; C15H12O5; UNII-HN5425SBF2; NSC 34875; HN5425SBF2; CHEBI:17846; AI3-23355; Flavanone, 4',5,7-trihydroxy-; (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; (-)-Naringenin; YSO1; EINECS 207-550-2; NSC-11855; 2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; CHEMBL9352; NARINGENIN, (-)-; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (2S)-; DTXSID1022392; (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one; 5,7,4'-Trihydroxyflavanone; (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone; (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one; 5,7-dihydroxy-2-(4-hydroxyphenyl)-4h-chroman-4-one; (-)-(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one; (R,S)-Naringenin; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one; NSC-34875; (S)-2,3-dihydo-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (S)-; SR-01000721771; (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one; 4',5,7-trihydroxyflavan-4-one; Nari; BE-14348A; 2uxu; 4deu; S-Dihydrogenistein; pelargidanon 1602; Spectrum_000247; 4eh3; NARINGENIN [MI]; Spectrum2_000325; Spectrum3_000567; Spectrum4_000124; Spectrum5_001423; NARINGENIN [INCI]; 4',5,7-triOH-Flavone; 4',5,7-Trihydroxyflavanon; SCHEMBL20570; BSPBio_001954; KBioGR_000508; KBioSS_000727; MLS000574861; BIDD:ER0116; DivK1c_000118; SPECTRUM1500746; SPBio_000329; 4',5, 7-Trihydroxyflavanone; DTXCID302392; BDBM23419; HMS500F20; KBio1_000118; KBio2_000727; KBio2_003295; KBio2_005863; KBio3_001454; FTVWIRXFELQLPI-ZDUSSCGKSA-N; NINDS_000118; (2S)-5,7,4'-trihydroxyflavone; AIDS001417; HMS2202M06; (2S)-4',5,7-trihydroxyflavanone; HY-N0100; TNP00287; (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one; CCG-38601; LMPK12140001; Phytochemistry 8: 127 (1969); s2394; AKOS016843490; CS-6421; DB03467; FS-4072; SDCCGMLS-0066570.P001; (2S)-4',5,7-trihydroxyflavan-4-one; IDI1_000118; NCGC00016457-01; NCGC00016457-02; NCGC00016457-03; NCGC00017346-01; NCGC00163598-01; AC-33954; CAS-480-41-1; Flavanone, 4',5,7-trihydroxy- (8CI); SMR000156272; FT-0617134; SW219329-1; C00509; EN300-303163; A827427; Q418374; Q-100666; SR-01000721771-3; SR-01000721771-4; BRD-K08832567-001-02-4; BRD-K08832567-001-06-5; 2,3-Dihydro-5,6-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one,2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (2S)-; 13308-00-4
Click to Show/Hide
|
||||
| Structure |
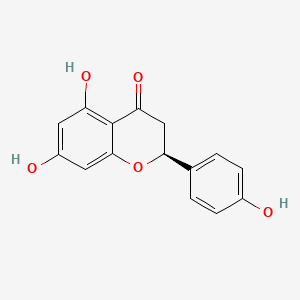 |
||||
| Formula |
C15H12O5
|
||||
| IUPAC Name |
(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one
|
||||
| Canonical SMILES |
C1C(OC2=CC(=CC(=C2C1=O)O)O)C3=CC=C(C=C3)O
|
||||
| InChI |
InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2/t13-/m0/s1
|
||||
| InChIKey |
FTVWIRXFELQLPI-ZDUSSCGKSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
20 Sprague Dawley (SD) rats (6-8 weeks, 200-220 g) were acquired from the Second Clinical College of Guangzhou University of Traditional Chinese Medicine, and were weighed, coded, and randomly assigned to experimental groups. Rats were divided into Sham group, MI/R group, MI/R +NAR (low dose, 10 mg/kg/d) group, and MI/R +NAR (high dose, 50 mg/kg/d) group. For MI/R model, rats were anaesthetized by intraperitoneal injection of 1% pentobarbital sodium (60 mg/kg) and then received mechanical ventilation from an animal ventilator after endotracheal intubation.
Click to Show/Hide
|
||||
| Response regulation | Naringenin alleviated MI/R-induced pathological damage, inflammation and lipid peroxidation in myocardial tissue of rats. NAR adjusted the NRF2 /System xc - /GPX4 axis and improved ferroptosis. In conclusion, NAR can alleviate myocardial ischemia-reperfusion injury by regulating the Nrf2/System xc-/GPX4 axis to inhibit ferroptosis. | ||||
References
