Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0329)
| Name |
Ulinastatin
|
||||
|---|---|---|---|---|---|
| Synonyms |
80499-32-7; 2,4-Dioxaspiro[5.5]undec-8-ene, 3-(2-furanyl)-; 80449-32-7; 3-(FURAN-2-YL)-2,4-DIOXASPIRO[5.5]UNDEC-8-ENE; Trypsin inhibitor (human urine urinastatin protein moiety); 3-(furan-2-yl)-2,4-dioxaspiro[5.5]undec-9-ene; 2,4-Dioxaspiro(5.5)undec-8-ene, 3-(2-furanyl)-; Trypsin inhibitor (human urine urinastatin protein moiety)80499-32-7; Trypsin inhibitor MR-20; MR-20 (Magnetic powder); Uti(68); Acid-stable protease inhibitor; Urinary trypsin inhibitor (68); MR 20; UNII-OR3S9IF86U; Uristatin; Mingin; Urinary trypsin inhibitor-like inhibitor (43); Trypsin inhibitor UTI; Ulinastatin [INN:JAN]; Trypsin inhibitor, mingin; Trypsin inhibitor, bikunin; Urinary trypsin inhibitor 1; 3-(2-Furanyl)-2,4-dioxaspiro(5.5)undec-8-ene; DTXSID801001247; BCP08067; AKOS025401879; AC-26827; HY-21646; D77201; 9-(2-furyl)-8,10-dioxaspiro[5.5]undec-3-ene; UTI
Click to Show/Hide
|
||||
| Status |
Phase 3
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
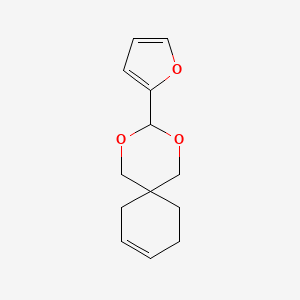 |
||||
| Formula |
C13H16O3
|
||||
| IUPAC Name |
3-(furan-2-yl)-2,4-dioxaspiro[5.5]undec-9-ene
|
||||
| Canonical SMILES |
C1CC2(CC=C1)COC(OC2)C3=CC=CO3
|
||||
| InChI |
InChI=1S/C13H16O3/c1-2-6-13(7-3-1)9-15-12(16-10-13)11-5-4-8-14-11/h1-2,4-5,8,12H,3,6-7,9-10H2
|
||||
| InChIKey |
ODVKSTFPQDVPJZ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response regulation | Ulinastatin plays a role in mitigation of Acetaminophen (APAP)-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response regulation | Ulinastatin plays a role in mitigation of APAP-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Heme oxygenase 1 (HMOX1)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response regulation | Ulinastatin (UT1) plays a role in mitigation of Acetaminophen (APAP)-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response regulation | Ulinastatin plays a role in mitigation of APAP-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute pancreatitis | ICD-11: DC31 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | 266-6 cells | Normal | Mus musculus | CVCL_3481 | |
| In Vivo Model |
For cerulein-induced acute pancreatitis, male mice (age, 8-10 wk) received 7 hourly intraperitoneal injections of 50 g/kg cerulein in sterile saline. Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8-10 wk) were fed a LieberDeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington,NJ).In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the LieberDeCarli ethanol liquid diet.
Click to Show/Hide
|
||||
| Response regulation | Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice. | ||||
References
