Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0245)
| Name |
Fraxetin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Fraxetin; 574-84-5; 7,8-Dihydroxy-6-methoxycoumarin; 7,8-Dihydroxy-6-methoxy-2H-chromen-2-one; 7,8-dihydroxy-6-methoxychromen-2-one; 2H-1-Benzopyran-2-one, 7,8-dihydroxy-6-methoxy-; UNII-CD3GD44O3K; 7,8-Dihydroxy-6-methoxy-2-benzopyrone; CD3GD44O3K; 7,8-Dihydroxy-6-methoxy-chromen-2-one; CHEMBL54909; CHEBI:5169; EINECS 209-376-2; 7,8-Dihydroxy-6-methoxy-2H-1-benzopyran-2-one; Fraxetol; 8-hydroxyscopoletin; Spectrum_001507; SpecPlus_000477; FRAXETIN [MI]; Spectrum2_001639; Spectrum3_001842; Spectrum4_001686; Spectrum5_000332; Oprea1_735469; SCHEMBL43472; BSPBio_003224; Fraxetin, analytical standard; KBioGR_001952; KBioSS_001987; MLS002207123; DivK1c_006573; SPECTRUM1504069; SPBio_001737; MEGxp0_000506; ACon0_001071; ACon1_000442; KBio1_001517; KBio2_001987; KBio2_004555; KBio2_007123; KBio3_002724; DTXSID00205992; HAVWRBANWNTOJX-UHFFFAOYSA-N; 7,8-dihydroxy-6-methoxy coumarin; KUC106681N; HY-N0580; TNP00177; Coumarin, 7,8-dihydroxy-6-methoxy; BDBM50206215; CCG-38759; MFCD00006873; s9503; STL564671; Coumarin, 7,8-dihydroxy-6-methoxy-; AKOS000277991; 7,8-Dihydroxy-6-methoxycoumarin, 98%; NCGC00017270-01; NCGC00017270-02; NCGC00017270-03; NCGC00017270-04; NCGC00017270-05; NCGC00096046-01; NCGC00096046-02; NCGC00169075-01; NCGC00169075-02; AC-34572; AS-67313; SMR000112323; KSC-11-207-12; CS-0009115; FT-0632418; 7,8-Dihydroxy-6-methoxy-2H-chromen-2-one #; A14554; C09265; SR-05000002449; Q-100662; SR-05000002449-1; BRD-K76587808-001-03-8; Q15410973; InChI=1/C10H8O5/c1-14-6-4-5-2-3-7(11)15-10(5)9(13)8(6)12/h2-4,12-13H,1H
Click to Show/Hide
|
||||
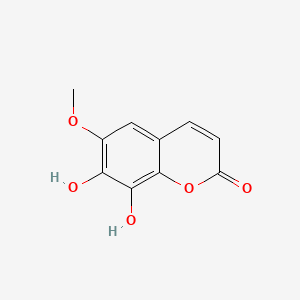
| Structure |
 |
||||
| Formula |
C10H8O5
|
||||
| IUPAC Name |
7,8-dihydroxy-6-methoxychromen-2-one
|
||||
| Canonical SMILES |
COC1=C(C(=C2C(=C1)C=CC(=O)O2)O)O
|
||||
| InChI |
InChI=1S/C10H8O5/c1-14-6-4-5-2-3-7(11)15-10(5)9(13)8(6)12/h2-4,12-13H,1H3
|
||||
| InChIKey |
HAVWRBANWNTOJX-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Acute myocardial infarction | ICD-11: BA41 | |||
| Responsed Regulator | RAC-alpha serine/threonine-protein kinase (AKT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Wistar rats (200-250 g) were obtained from Slac Laboratory Animal Center (Shanghai, China) and kept in cages. The rats were anesthetized with 1% pentobarbital and then lied on its back. Thereafter, the left precordial area of the rats were shaved and disinfected, followed by trachea intubation for artificial ventilation. After the left thoracotomy, the heart was fully exposed and the left coronary artery (LAD) was ligated with a 6-0 prolene suture at 2-3 mm from its origin between the pulmonary artery conus and the left atrial appendage. After 30 min, the suture was gently removed to allow reperfusion for 2 h.
Click to Show/Hide
|
||||
| Response regulation | Fraxetin activated phosphorylation of AKT and Nrf2 nuclear accumulation in Myocardial infarction in vivoandin vitromodels. Moreover, Fra reduced the activity of serum LDH, the accumulation of iron and the MDA level, and increased GSH and glutathione peroxidase 4 (GPX4) in rats with MI. | ||||
