Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0036)
| Name |
Ibuprofen
|
||||
|---|---|---|---|---|---|
| Synonyms |
ibuprofen; 15687-27-1; 2-(4-Isobutylphenyl)propanoic acid; Motrin; Brufen; Advil; Nurofen; Nuprin; Dolgit; Liptan; Medipren; Anflagen; Buburone; Butylenin; Ibumetin; Ibuprocin; Lamidon; Ebufac; Rufen; Mynosedin; Roidenin; Andran; Apsifen; Bluton; Epobron; Haltran; Nobfen; Nobgen; Trendar; Adran; PediaProfen; 2-[4-(2-methylpropyl)phenyl]propanoic acid; Brufort; Ibuprohm; Inabrin; Nobfelon; Pantrop; Rebugen; Suspren; Tabalon; Burana; Anco; Urem; (RS)-Ibuprofen; Ibu-slo; Brufanic; Napacetin; Ibufen; Ibuprin; Profen; Ibren; Midol; 4-Isobutylhydratropic acid; Cap-Profen; Tab-Profen; Ibu-Tab; Novoprofen; Proartinal; Amersol; Dibufen; Dolocyl; Lidifen; Optifen; Ostofen; p-Isobutylhydratropic acid; Panafen; Paxofen; Proflex; Quadrax; Uprofen; Carol; Rafen; Cobo; Ifen; (+-)-Ibuprofen; (+/-)-Ibuprofen; Artril 300; duralbuprofen; Balkaprofen; Betaprofen; Butacortelone; Daiprophen; Ibuprofeno; Ibuprofenum; Jenaprofen; Algofen; Amelior; Amibufen; Antagil; Antalfene; Antarene; Antiflam; Artofen; Bruflam; Bufigen; Bukrefen; Buracaps; Citalgan; Combiflam; Dansida; Dentigoa; Dignoflex; Dolgirid; Dolmaral; Dolofen; Dolofin; Dolofort; Dologel; Dolomax; Doloren; Doltibil; Dorival; Duobrus; Dysdolen; Easifon; Exneural; Femadon; Femafen; Femapirin; Femidol; Fenspan; Fibraflex; Gelufene; Gynofug; Ibubest; Ibubeta; Ibucasen; Ibudolor; Ibuflamar; Ibugesic; Ibuhexal; Ibulagic; Ibuleve; Ibulgan; Ibumerck; Ibupirac; Junifen; Kratalgin; Lebrufen; Librofem; Malafene; Manypren; Mensoton; Neobrufen; Nerofen; Noalgil; Nobafon; Noritis; Novadol; Novogent; Nuprilan; Opturem; Oralfene; Ostarin; Paduden; Perofen; Ranofen; Relcofen; Rhinadvil; Sadefen; Salivia; Seclodin; Sednafen; Seklodin; Seskafen; Siyafen; Solpaflex; Sugafen; Suprafen; Syntofene; Tatanal; Unipron; Alaxan; Anafen; Artril; Bloom; Brofen; Bufeno; Bupron; Cesra; Cunil; Dalsy; Dolibu; Dolven; Duafen; Emflam; Eputex; Ergix; Faspic; Fenbid; Fendol; Gofen; Grefen; Ibudol; Ibufug; Ibugel; Ibugen; Ibular; Ibulav; Ibumed; Ibusal; Ibutid; Inflam; Inoven; Ipren; Irfen; Isodol; Kesan; Lopane; Melfen; Midol 200; Moment; Narfen; Ozonol; Provon; Rofen; Rufin; Rupan; Stelar; Tempil; Tofen; Tonal; Upfen; Zofen; Drin; Ibol; Pediatric Advil; 4-Isobutyl-alpha-methylphenylacetic acid; Apo-Ibuprofen; Brufen Retard; Deep Relief; Dolo-Dolgit; Ibu-Attritin; Neo-Helvagit; Novo-Profen; Ibu-slow; Neo-Mindol; Dolofen-F; Donjust B; Nagifen-D; Dura-Ibu; Children's Advil; alpha-(4-Isobutylphenyl)propionic acid; Motrin IB; Children's Motrin; Togal N; ACHES-N-PAIN; Dularbuprofen; Caldolor; Ibu-Tab 200; Ibuprofene; Ibuprophen; Pedea; Children's Ibuprofen; IP-82; Kontagripp Mono; Tabalon 400; Novo Dioxadol; Schmerz-Dolgit; Junior Strength Advil; Brufen 400; Emflam-200; DOLO PUREN; Hemagene Tailleur; Junior Strength Motrin; Act-3; Ak+C2278tren; Advil Liqui-Gels; Adex 200; Codral Period Pain; Hydratropic acid, p-isobutyl-; Junior Strength Ibuprofen; (+-)-Ibuprophen; Children's Elixsure; RD 13621; Am-Fam 400; (4-Isobutylphenyl)-alpha-methylacetic acid; 58560-75-1; IB-100; Alivium; (+-)-p-Isobutylhydratropic acid; Advil migraine; p-Isobutyl-2-phenylpropionic acid; U-18,573; Perrigo ibuprofen; (+-)-2-(p-Isobutylphenyl)propionic acid; alpha-(p-isobutylphenyl)propionic acid; VUFB 9649; MOTRIN MIGRAINE PAIN; alpha-p-Isobutylphenylpropionic acid; UCB 79171; CCRIS 3223; CHEBI:5855; (+-)-alpha-Methyl-4-(2-methylpropyl)benzeneacetic acid; CHILDREN'S ADVIL-FLAVORED; HSDB 3099; UNII-WK2XYI10QM; alpha-Methyl-4-(2-methylpropyl)benzeneacetic acid; Advil Migraine Liqui-Gels; WK2XYI10QM; (+/-)-p-Isobutylhydratropic acid; 2-(p-Isobutylphenyl)propionic acid; EINECS 239-784-6; Midol Liquid Gels; Benzeneacetic acid, .alpha.-methyl-4-(2-methylpropyl)-; NSC 256857; NSC-256857; BRN 2049713; U 18573; U-18573; DTXSID5020732; Ibu; 2-(4-ISOBUTYLPHENYL)PROPIONIC ACID; M01AE01; MFCD00010393; NSC256857; CHEMBL521; 2-(4-Isobutyl-phenyl)-propionic acid; Acide (isobutyl-4-phenyl)-2 propionique; Benzeneacetic acid, alpha-methyl-4-(2-methylpropyl)-; DTXCID90732; alpha-Methyl-4-(isobutyl)phenylacetic acid; (+/-)-2-(p-isobutylphenyl)propionic acid; MLS000069733; Esprenit; R.D. 13621; Zafen; EC 239-784-6; alpha-(4-Isobutylphenyl)propionate; COMBUNOX COMPONENT IBUPROFEN; REPREXAIN COMPONENT IBUPROFEN; (.+-.)-p-Isobutylhydratropic acid; VICOPROFEN COMPONENT IBUPROFEN; IBUPROFEN COMPONENT OF COMBUNOX; SINE-AID IB COMPONENT IBUPROFEN; IBUPROFEN COMPONENT OF REPREXAIN; IBUPROFEN COMPONENT OF VICOPROFEN; .alpha.-(p-isobutylphenyl)propionic acid; NCGC00015529-09; .alpha.-(4-Isobutylphenyl)propionic acid; Acide (isobutyl-4 phenyl)-2 propionique; SMR000058184; IBUPROFEN COMPONENT OF SINE-AID IB; 4-Isobutyl-.alpha.-methylphenylacetic acid; ADVIL ALLERGY SINUS COMPONENT IBUPROFEN; IBUPROFEN (MART.); IBUPROFEN [MART.]; CHILDREN'S ADVIL COLD COMPONENT IBUPROFEN; ADVIL CONGESTION RELIEF COMPONENT IBUPROFEN; CHILDREN'S MOTRIN COLD COMPONENT IBUPROFEN; IBUPROFEN COMPONENT OF ADVIL ALLERGY SINUS; Advil, Children's; .alpha.-Methyl-4-(2-methylpropyl)benzeneacetic acid; IBUPROFEN COMPONENT OF ADVIL CONGESTION RELIEF; IBUPROFEN COMPONENT OF CHILDREN'S ADVIL COLD; IBUPROFEN COMPONENT OF CHILDREN'S MOTRIN COLD; IBUPROFEN (EP MONOGRAPH); IBUPROFEN (USP IMPURITY); IBUPROFEN [EP MONOGRAPH]; IBUPROFEN [USP IMPURITY]; IBUPROFEN (USP MONOGRAPH); IBUPROFEN [USP MONOGRAPH]; Actiprofen; Midol IB Cramp Relief; Benzeneacetic acid, a-methyl-4-(2-methylpropyl)-, (A+/-)-; Genpril; Codral; Bayer Select Pain Relief; Pedia-Profen; Ibuprofene [INN-French]; Ibuprofenum [INN-Latin]; Novogent N; Apsifen-F; Racemic ibuprofen; Ibuprofeno [INN-Spanish]; 2-(4-isobutylphenyl)propanoate; Motrin IB Gelcaps; CAS-15687-27-1; Motrin (TN); 2-(4-(2-methylpropyl)phenyl)propanoic acid; Advil (TN); (4-isobutylphenyl)-alpha-methylacetate; Children's Elixsure IB; SR-01000000214; alpha-methyl-4-(2-methylpropyl)benzeneacetate; Ibupril; Ibuprox; Aktren; Ibux; rac Ibuprofen; (+/-)-2-(4-Isobutylphenyl)Propanoic Acid; CDT-ibuprofen; MFCD00069289; Nurofen Meltlets; (y)-Ibuprofen; Ibuprofen,(S); (+) ibuprofen; Ibuprofen (Advil); Acide (isobutyl-4-phenyl)-2 propionique [French]; (?)-Ibuprofen; Ibuprofen [USAN]; EPROBRON; p-Isobutylhydratropate; Ibuprofen [USAN:USP:INN:BAN:JAN]; Combunox (Salt/Mix); (A+/-)-Ibuprofen; INFANT'S ADVIL; Spectrum_000849; IBUPROFEN [INN]; IBUPROFEN [JAN]; Opera_ID_554; IBUPROFEN [MI]; IBUPROFEN [HSDB]; IBUPROFEN [INCI]; Spectrum2_000129; Spectrum3_000465; Spectrum4_000015; Spectrum5_000862; IBUPROFEN [VANDF]; WLN: QVY&R DIY; CBMicro_005634; Epitope ID:139973; p-isobutyl-hydratropic acid; I 4883; Cambridge id 5152402; IBUPROFEN [USP-RS]; IBUPROFEN [WHO-DD]; IBUPROFEN [WHO-IP]; SCHEMBL3001; Lopac0_000691; BSPBio_002170; Ibuprofen - Adooq Bioscience; IBUPROFEN [EMA EPAR]; KBioGR_000389; KBioSS_001329; MLS001146965; p-Isobutyl-2-phenylpropionate; BIDD:GT0050; DivK1c_000887; SPECTRUM1500347; Ibuprofen, >=98% (GC); SPBio_000178; Ibuprofen (JP17/USP/INN); Ibuprofen, 1mg/ml in Methanol; alpha-p-Isobutylphenylpropionate; GTPL2713; Motrin IB Gelcaps (Salt/Mix); IBUPROFEN [ORANGE BOOK]; 2-p-isobutylphenylpropionic acid; HEFNNWSXXWATRW-UHFFFAOYSA-; HMS502M09; KBio1_000887; KBio2_001329; KBio2_003897; KBio2_006465; KBio3_001390; Advil Cold & Sinus (Salt/Mix); C01CB16; G02CC01; M02AA13; R02AX02; Ibuprofen 1.0 mg/ml in Methanol; NINDS_000887; Sine-Aid IB Caplets (Salt/Mix); Benzeneacetic acid, alpha-methyl-4-(2-methylpropyl), (+-)-; HMS1920F15; HMS2089P05; HMS2091N03; HMS2230N04; HMS3259G05; HMS3262K03; HMS3372M09; HMS3649M11; HMS3651A15; HMS3884I04; Pharmakon1600-01500347; IBUPROFENUM [WHO-IP LATIN]; BCP10423; BCP20325; BCP25225; ZAG-1701; ( inverted question mark)-Ibuprofen; Children's Elixsure IB (Salt/Mix); Tox21_110170; Tox21_201384; Tox21_302829; Tox21_500691; 1189866-35-0 (unlabeled); 2-(4'-isobutylphenyl)propionic acid; 2-(4-isobutylphenyl) propanoic acid; 2-(4-isobutylphenyl) propionic acid; 2-(4-isobutylphenyl)-propionic acid; BBL010660; BDBM50009859; CCG-38947; COMBOGESIC COMPONENT IBUPROFEN; NSC757073; s1638; STK177358; (.+/-.)-p-Isobutylhydratropic acid; 2-(4-isobutylphenyl)-propionoic acid; 2-(4'-isobutylphenyl)-propionic acid; AKOS003237488; AKOS016340658; Propanoic acid, 2-(4-isobutylphenyl); Tox21_110170_1; ( inverted exclamation markA)-Ibuprofe; CS-1931; DB01050; KS-5029; LP00691; NC00458; NSC-757073; SB19113; SDCCGSBI-0050669.P005; ST-1482; alpha-(4-isobutylphenyl)-propionic acid; alpha-(p-isobutylphenyl)-propionic acid; IBUPROFEN COMPONENT OF COMBOGESIC; IDI1_000887; 2-(4-Isobutylphenyl)propionic Acid-d3;; NCGC00015529-04; NCGC00015529-05; NCGC00015529-06; NCGC00015529-07; NCGC00015529-08; NCGC00015529-10; NCGC00015529-12; NCGC00015529-13; NCGC00015529-14; NCGC00015529-15; NCGC00015529-18; NCGC00015529-32; NCGC00089819-02; NCGC00089819-03; NCGC00089819-04; NCGC00089819-05; NCGC00089819-06; NCGC00089819-07; NCGC00256416-01; NCGC00258935-01; NCGC00261376-01; AC-11312; BI166241; HY-78131; NCI60_002065; SY046826; Ibuprofen, Vetec(TM) reagent grade, 97%; SBI-0050669.P004; .alpha.-2-(p-Isobutylphenyl)propionic acid; Ibuprofen, meets USP testing specifications; (.+-.)-2-(p-Isobutylphenyl)propionic acid; 4-Isobutylphenyl)-.alpha.-methylacetic acid; AM20060782; BB 0258487; EU-0100691; FT-0601629; FT-0642971; FT-0655194; FT-0670257; FT-0670258; I0415; SW203738-2; (.+/-.)-2-(p-Isobutylphenyl)propionic acid; Ibuprofen, VETRANAL(TM), analytical standard; (r/s)-alpha-methyl-4-isobutylphenylacetic acid; C01588; D00126; EN300-120638; AB00052020-16; AB00052020-17; AB00052020_18; AB00052020_19; A831926; Q186969; (2RS)-2-(4-(2-Methylpropyl)phenyl)propanoic acid; J-009349; SR-01000000214-2; SR-01000000214-4; BRD-A17655518-001-02-0; BRD-A17655518-001-12-9; SR-01000000214-13; F2173-0233; Ibuprofen, British Pharmacopoeia (BP) Reference Standard; Pharmaceuticals Mixture 327 200 microg/mL in Methanol; Z1695709473; (+/-)-alpha-methyl-4-(2-methylpropyl)benzeneacetic acid; Ibuprofen, European Pharmacopoeia (EP) Reference Standard; Ibuprofen, United States Pharmacopeia (USP) Reference Standard; Benzeneacetic acid, .alpha.-methyl-4-(2-methylpropyl), (.+/-.)-; BENZENEACETIC ACID, .ALPHA.-METHYL-4-(2-METHYLPROPYL), (+/-)-; Dexibuprofen;(S)-Ibuprofen; L 669455; L-669,455, MK 233; MK-233; Ibuprofen, Pharmaceutical Secondary Standard; Certified Reference Material; Ibuprofen for peak identification, European Pharmacopoeia (EP) Reference Standard; 139466-08-3; InChI=1/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
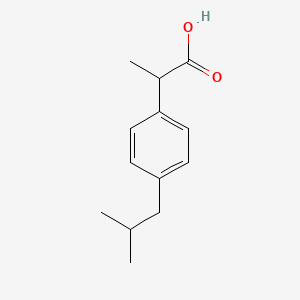 |
||||
| Formula |
C13H18O2
|
||||
| IUPAC Name |
2-[4-(2-methylpropyl)phenyl]propanoic acid
|
||||
| Canonical SMILES |
CC(C)CC1=CC=C(C=C1)C(C)C(=O)O
|
||||
| InChI |
InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
|
||||
| InChIKey |
HEFNNWSXXWATRW-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
In the intracranial glioma model, U87MG cells (5 x 105) were intracerebrally injected into the left side (bregma: 1 mm; lateral: 2 mm; ventral: 3 mm) of the brains of nude mice. Two weeks after tumor cell transplantation, mouse brains were scanned to detect tumor formation using a 3.0-T scanner (GE Signa HD MRI Systems). Then, mice were divided randomly into two groups (n = 6/group) and treated with vehicle control (PBS), oribuprofen (20 mg/kg), in 100 ul of PBS given i.p. 1x/day, 5 days/week.
Click to Show/Hide
|
||||
| Response regulation | Ibuprofen could induce ferroptosis of glioblastoma cells via downregulation of Nrf2 signaling pathway and is a potential drug for glioma treatment. All the data suggested that Nrf2 could regulate the expression of GPX4 and SLC7A11 in glioma cells. | ||||
