Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0041)
| Name |
Pioglitazone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Pioglitazone; 111025-46-8; Actos; Glustin; 105355-27-9; Zactos; Pioglitazona; Pioglitazonum; Pioglitazonum [INN-Latin]; 5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione; Pioglitazona [INN-Spanish]; AD-4833; U 72107; Pioglitazone [BAN:INN]; Pioglitazone (Actos); 5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione; pioglitazone (INN); 105390-47-4; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; CHEBI:8228; C19H20N2O3S; U-72107; 5-[4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]thiazolidine-2,4-dione; Actos (TN); 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione; 2,4-thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-; X4OV71U42S; 5-[4-[2-(5-ETHYL-2-PYRIDYL)ETHOXY]BENZYL]-2,4-THIAZOLIDINEDIONE; 5-({4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}methyl)-1,3-thiazolidine-2,4-dione; MFCD00865504; PIOGLITAZONE [INN]; 5-((4-(2-(5-Ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione; Pioglitazone [INN:BAN]; Piozone; Actost; Pioglu; 2,4-Thiazolidinedione, 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-; HSDB 7322; UNII-X4OV71U42S; 5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4-thiazolidinedione; SR-01000763737; 5-(4-(2-(5-ETHYL-2-PYRIDYL)ETHOXY)BENZYL)THIAZOLIDINE-2,4-DIONE; 2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-, (+/-)-; [( inverted exclamation markA)-5-[[4-[2-(5-ethyl-2-pyridinyl) ethoxy] phenyl] methyl]-2,4-] thiazolidinedione monohydrochlorid; 2,4-THIAZOLIDINEDIONE, 5-((4-(2-(5-ETHYL-2-PYRIDINYL)ETHOXY)PHENYL)METHYL)-, (+/-)-; Pioglitazone-[d4]; Pioglitazone- Bio-X; HS-0047; Spectrum_001623; Spectrum2_001679; Spectrum3_001002; Spectrum4_001130; Spectrum5_001480; Spectrum5_002067; PIOGLITAZONE [MI]; PIOGLITAZONE [HSDB]; PIOGLITAZONE [IARC]; SCHEMBL4121; PIOGLITAZONE [VANDF]; BSPBio_002723; KBioGR_001619; KBioSS_002103; MLS006011848; PIOGLITAZONE [WHO-DD]; SPBio_001897; GTPL2694; PIOGLITAZONE [EMA EPAR]; DTXSID3037129; KBio2_002103; KBio2_004671; KBio2_007239; KBio3_001943; HYAFETHFCAUJAY-UHFFFAOYSA-N; HMS2089H14; HMS3651D09; HMS3712E16; HMS3884L10; Pharmakon1600-01504401; BCP26474; BBL029068; BDBM50103521; HB4139; NSC758876; s2590; STL309607; STL373406; (+/-)-5-[p-[2-(ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]thiazolidine-2,4-dione; AKOS015894953; AKOS022109420; AC-1021; CCG-220107; CS-1700; DB01132; SB17323; (+/-)-5-[[4-[2-(5-Ethyl-2-pyridinyl)-ethoxy]phenyl]methyl]-2,4-thiazolidinedione; 2,4-Thiazolidinedione, 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-, (+-)-; NCGC00163128-01; NCGC00163128-02; NCGC00163128-03; NCGC00163128-04; NCGC00163128-05; NCGC00163128-06; NCGC00163128-07; BP164273; HY-13956; SMR002204015; SY017473; SBI-0206791.P001; FT-0601906; FT-0645030; SW197561-3; C07675; D08378; EN300-117258; AB00698454-10; AB00698454_11; AB00698454_12; AB00698454_13; A802277; Q417765; J-002506; J-516181; SR-01000763737-5; BRD-A48430263-003-02-4; BRD-A48430263-003-06-5; Z1501480426; 5-[4-[2-(5-ethyl-2-pyridyl) ethoxy]benzyl]-2,4-thiazolidinedione; 5-[4-[2-(5-ethyl-2-pyridyl)eth-oxy]benzyl]-2,4-thiazolidinedione; 5-{4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl}-2,4thiazolidinedione; 5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)-thiazolidine-2,4-dione; 5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl-2,4-thiazolidinedione; 5-[[4-[2-(5-ethyl-2-pyridyl)ethoxy] phenyl]methyl]-2,4-thiazolidinedione; (+/-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione; (+/-)-5-(P-(2-(5-ETHYL-2-PYRIDYL)ETHOXY)BENZYL)-2,4-THIAZOLIDINEDIONE; (5S)-5-[[4-[2-(5-ethyl-2-pyridyl)ethoxy]phenyl]methyl]thiazolidine-2,4-dione;Pioglitazone; (RS)-5-(4-(2-(5-ETHYLPYRIDIN-2-YL)ETHOXY)BENZYL)THIAZOLIDINE-2,4-DIONE; 2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]- (9CI); 5-[[4-[2-[(5-ethyl-2-pyridyl)]ethoxy]phenyl]methyl]thiazolidine- 2,4-dione; 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-4-hydroxy-1,3-thiazol-2(5H)-one; [()-5-[[4-[2-(5-ethyl-2-pyridinyl) ethoxy] phenyl] methyl]-2,4-] thiazolidinedione monohydrochlorid; 198077-89-3
Click to Show/Hide
|
||||
| Structure |
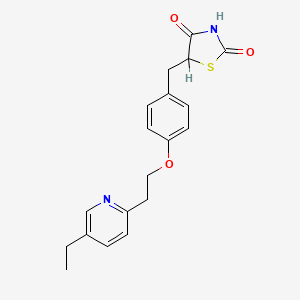 |
||||
| Formula |
C19H20N2O3S
|
||||
| IUPAC Name |
5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
|
||||
| Canonical SMILES |
CCC1=CN=C(C=C1)CCOC2=CC=C(C=C2)CC3C(=O)NC(=O)S3
|
||||
| InChI |
InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23)
|
||||
| InChIKey |
HYAFETHFCAUJAY-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Responsed Regulator | Peroxisome proliferator-activated receptor gamma (PPARG) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPNCs (Rat primary nerve cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
The rats underwent surgery using an ultraclean table and were fixed in a stereotaxic frame. The scalp was opened to expose the anterior brain region. A dental drill was used to drill a 1-mm-diameter hole in the skull surface. Blood (100 ul) was collected from the rat tail vein and injected into the rat striatum with a microsyringe (stereotaxic coordinates; 2 mm lateral to the midline, 0.2 mm posterior to bregma, and 5.5 mm deep below the skull). First, 60 ul of autogenous blood were injected at a rate of 2 ul/min, and the next 40 ul of blood were injected at 5 ul/min. Finally, the needle was left for 10 min before being removed.
Click to Show/Hide
|
||||
| Response regulation | Pioglitazone (PDZ), a PPAR agonist, promotes Gpx4 expression through the interaction between PPAR and the Nrf2 pathway, inhibits ferroptosis of neurons after intracerebral hemorrhage (ICH), and promotes the recovery of neural function. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Responsed Regulator | Peroxisome proliferator-activated receptor gamma (PPARG) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPNCs (Rat primary nerve cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
The rats underwent surgery using an ultraclean table and were fixed in a stereotaxic frame. The scalp was opened to expose the anterior brain region. A dental drill was used to drill a 1-mm-diameter hole in the skull surface. Blood (100 ul) was collected from the rat tail vein and injected into the rat striatum with a microsyringe (stereotaxic coordinates; 2 mm lateral to the midline, 0.2 mm posterior to bregma, and 5.5 mm deep below the skull). First, 60 ul of autogenous blood were injected at a rate of 2 ul/min, and the next 40 ul of blood were injected at 5 ul/min. Finally, the needle was left for 10 min before being removed.
Click to Show/Hide
|
||||
| Response regulation | Pioglitazone (PDZ), a PPAR agonist, promotes Gpx4 expression through the interaction between PPAR and the Nrf2 pathway, inhibits ferroptosis of neurons after intracerebral hemorrhage (ICH), and promotes the recovery of neural function. | ||||
