Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0184)
| Name |
Dexmedetomidine
|
||||
|---|---|---|---|---|---|
| Synonyms |
DEXMEDETOMIDINE; 113775-47-6; Dexmedetomidinum; MPV 1440; Precedex; 4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole; 5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole; MPV-1440; Dexmedetomidina; (+)-4-((S)-alpha,2,3-Trimethylbenzyl)imidazole; Medetomidine, (s)-; (S)-5-(1-(2,3-Dimethylphenyl)ethyl)-1H-imidazole; Precedex (TN); CHEMBL778; 67VB76HONO; CHEBI:4466; 113775-47-6 (free base); 1H-Imidazole, 4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-; 4-[(1~{S})-1-(2,3-dimethylphenyl)ethyl]-1~{H}-imidazole; (S)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole; UNII-67VB76HONO; Dexmedetomidine (USAN/INN); Dexmedetomidinum [INN-Latin]; Dexmedetomidina [INN-Spanish]; MFCD00880557; Dexmedetomidine [USAN:INN:BAN]; Dexdor (TN); Tocris-2023; NCGC00025347-01; (S)-4-(1-(2,3-dimethylphenyl)ethyl)-1H-imidazole; DEXMEDETOMIDINE [MI]; SCHEMBL26433; DEXMEDETOMIDINE [INN]; GTPL521; DEXMEDETOMIDINE [USAN]; DEXMEDETOMIDINE [VANDF]; (+)-(S)-4-[1-(2,3-dimethylphenyl) ethyl]-1H -imidazole monohydrochloride; DEXMEDETOMIDINE [WHO-DD]; DexmedetomidineHclC13H16N2.Hcl; DTXSID10873388; TPU 006; CUHVIMMYOGQXCV-NSHDSACASA-N; HMS3885M07; DEXMEDETOMIDINE [GREEN BOOK]; BDBM50085683; s3075; AKOS025149503; AKOS026750524; CCG-266586; DB00633; Igalmi (dexmedetomidine sublingual film); NCGC00371080-02; NCGC00371080-09; AS-68685; HY-12719; CS-0012295; SW219607-1; C07450; D00514; EN300-127736; AB01566872_01; AB01566872_02; Q412133; 4-[(1S)-1-(2,3-Dimethylphenyl)ethyl]-1H-imidazol; 4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-3H-imidazole; 1H-Imidazole, 5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-; 4-[(s)-1-(2,3-dimethyl-phenyl)-ethyl]-1h-imidazole; (+)-4-((S)-.ALPHA.,2,3-TRIMETHYLBENZYL)IMIDAZOLE
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
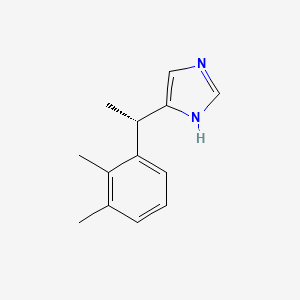 |
||||
| Formula |
C13H16N2
|
||||
| IUPAC Name |
5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole
|
||||
| Canonical SMILES |
CC1=C(C(=CC=C1)C(C)C2=CN=CN2)C
|
||||
| InChI |
InChI=1S/C13H16N2/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13/h4-8,11H,1-3H3,(H,14,15)/t11-/m0/s1
|
||||
| InChIKey |
CUHVIMMYOGQXCV-NSHDSACASA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | Transcription factor E2F7 (E2F7) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SNU-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0099 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Female BALB/c nude mice (4-6 weeks old) were obtained from Beijing Institute of Life Sciences (Beijing, China) and the mice were maintained under the standard conditions. AGS cells (2 x 106 cells/mL) were suspended in 100 ul of PBS and were subcutaneously injected in the right flank of mice. After 1 week, mice were divided into four groups (n = 5): Ctrl, 0.5 ug/kg, 1.0 ug/kg, and 2.0 ug/kg groups. The mice were intraperitoneally injected with DEX once a day for 15 days. Mice in the control group were injected with the same amount of normal saline. Tumor size was measured every 2 days and calculated with the formula: 0.5 x length x width2. After the last DEX injection was completed, mice were euthanized with sodium pentobarbital (100 mg/kg) and then sacrificed by decapitation. The tumor tissues were isolated and weighted. Immunohistochemistry for Ki67 and TUNEL assay were performed on paraffin-embedded xenograft tumor tissue sections.
Click to Show/Hide
|
||||
| Response regulation | The current work studied the role of Dexmedetomidine (DEX) in gastric cancer cells and discovered that DEX suppressed GC growth by causing ferroptosis. Furthermore, the circ0008035/miR-302a/E2F7 axis was involved in DEX-induced ferroptotic cell death in GC. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | Circ_0008035 (circRNA) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SNU-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0099 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Female BALB/c nude mice (4-6 weeks old) were obtained from Beijing Institute of Life Sciences (Beijing, China) and the mice were maintained under the standard conditions. AGS cells (2 x 106 cells/mL) were suspended in 100 ul of PBS and were subcutaneously injected in the right flank of mice. After 1 week, mice were divided into four groups (n = 5): Ctrl, 0.5 ug/kg, 1.0 ug/kg, and 2.0 ug/kg groups. The mice were intraperitoneally injected with DEX once a day for 15 days. Mice in the control group were injected with the same amount of normal saline. Tumor size was measured every 2 days and calculated with the formula: 0.5 x length x width2. After the last DEX injection was completed, mice were euthanized with sodium pentobarbital (100 mg/kg) and then sacrificed by decapitation. The tumor tissues were isolated and weighted. Immunohistochemistry for Ki67 and TUNEL assay were performed on paraffin-embedded xenograft tumor tissue sections.
Click to Show/Hide
|
||||
| Response regulation | The current work studied the role of Dexmedetomidine (DEX) in Gastric cancer (GC) cells and discovered that DEX suppressed GC growth by causing ferroptosis. Furthermore, the circ0008035/miR-302a/E2F7 axis was involved in DEX-induced ferroptotic cell death in GC. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | hsa-mir-302a (Precursor RNA) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SNU-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0099 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Female BALB/c nude mice (4-6 weeks old) were obtained from Beijing Institute of Life Sciences (Beijing, China) and the mice were maintained under the standard conditions. AGS cells (2 x 106 cells/mL) were suspended in 100 ul of PBS and were subcutaneously injected in the right flank of mice. After 1 week, mice were divided into four groups (n = 5): Ctrl, 0.5 ug/kg, 1.0 ug/kg, and 2.0 ug/kg groups. The mice were intraperitoneally injected with DEX once a day for 15 days. Mice in the control group were injected with the same amount of normal saline. Tumor size was measured every 2 days and calculated with the formula: 0.5 x length x width2. After the last DEX injection was completed, mice were euthanized with sodium pentobarbital (100 mg/kg) and then sacrificed by decapitation. The tumor tissues were isolated and weighted. Immunohistochemistry for Ki67 and TUNEL assay were performed on paraffin-embedded xenograft tumor tissue sections.
Click to Show/Hide
|
||||
| Response regulation | The current work studied the role of Dexmedetomidine (DEX) in Gastric cancer (GC) cells and discovered that DEX suppressed GC growth by causing ferroptosis. Furthermore, the circ0008035/miR-302a/E2F7 axis was involved in DEX-induced ferroptotic cell death in GC. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Sepsis | ICD-11: 1G40 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mVTs (Mouse ventricular tissues) | ||||
| In Vivo Model |
A total of 32 male C57BL/6 mice (25 g, 8 weeks old) were obtained from the Guangdong Medical Lab Animal Center and housed in the Laboratory Animal Service Center (Jinan University, Guangdong, China). Mice were anesthetized with isoflurane (RWD Life Science) inhalation at the concentration of 2.5% for anesthetic induction and then at 1% for anesthetic maintenance until the end of the CLP. During the experiment, the body temperature was kept at 36-38 with a heating pad. Anesthetized mice were subjected to midline laparotomy. The cecum was carefully separated to avoid blood vessels damage and the cecum was identified and punctured twice with a 22-gauge needle. Then, the abdominal cavity was closed with two epithelium layers, followed by a normal saline injection subcutaneously for resuscitation before mice were returned to the cage.
Click to Show/Hide
|
||||
| Response regulation | The attenuation of sepsisinduced HO1 overexpression and iron concentration, and the reduction of ferroptosis via enhancing GPX4, may be the major mechanisms via which Dexmedetomidine alleviates sepsis induced myocardial cellular injury. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Driver/Suppressor | ||||
| Responsed Disease | Sepsis | ICD-11: 1G40 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mVTs (Mouse ventricular tissues) | ||||
| In Vivo Model |
A total of 32 male C57BL/6 mice (25 g, 8 weeks old) were obtained from the Guangdong Medical Lab Animal Center and housed in the Laboratory Animal Service Center (Jinan University, Guangdong, China). Mice were anesthetized with isoflurane (RWD Life Science) inhalation at the concentration of 2.5% for anesthetic induction and then at 1% for anesthetic maintenance until the end of the CLP. During the experiment, the body temperature was kept at 36-38 with a heating pad. Anesthetized mice were subjected to midline laparotomy. The cecum was carefully separated to avoid blood vessels damage and the cecum was identified and punctured twice with a 22-gauge needle. Then, the abdominal cavity was closed with two epithelium layers, followed by a normal saline injection subcutaneously for resuscitation before mice were returned to the cage.
Click to Show/Hide
|
||||
| Response regulation | The attenuation of sepsisinduced HO1 overexpression and iron concentration, and the reduction of ferroptosis via enhancing GPX4, may be the major mechanisms via which Dexmedetomidine alleviates sepsis induced myocardial cellular injury. | ||||
References
