Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0034)
| Name |
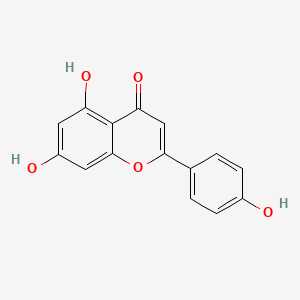
Apigenin
|
||||
|---|---|---|---|---|---|
| Synonyms |
apigenin; 520-36-5; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; Versulin; Apigenol; 4',5,7-Trihydroxyflavone; Chamomile; Spigenin; Apigenine; C.I. Natural Yellow 1; Pelargidenon 1449; 5,7,4'-Trihydroxyflavone; 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; NSC 83244; 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-; UCCF 031; CCRIS 3789; FLAVONE, 4',5,7-TRIHYDROXY-; CHEBI:18388; EINECS 208-292-3; MFCD00006831; NSC-83244; UNII-7V515PI7F6; APEGENIN; BRN 0262620; 8002-66-2; Chamomile oil, german; DTXSID6022391; HSDB 7573; 7V515PI7F6; CHEMBL28; PELARGIDENON-1449; CI NATURAL YELLOW 1; DTXCID902391; UCCF-031; 5-18-04-00574 (Beilstein Handbook Reference); NSC83244; CAS-520-36-5; LY-080400; 4',5,7-Trihydroxyflavone;Apigenol;C.I. Natural Yellow 1; SMR000326850; 4?5,7-Trihydroxyflavone; SR-01000075663; Pelargidenone; 4der; 4dgm; 4hkk; Naringenin, 18; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4',5,7-Trihydroxyflavone; Pelargidenon 1449;; Prestwick_719; Apigenin, 13; Tocris-1227; 3cf9; ST056301; APIGENIN [HSDB]; APIGENIN [INCI]; 4',7-Trihydroxyflavone; APIGENIN [MI]; BiomolKI_000078; Prestwick0_000414; Prestwick1_000414; Prestwick2_000414; Prestwick3_000414; Spectrum2_000428; Spectrum3_001882; Spectrum4_001999; Lopac-A-3145; APIGENIN [USP-RS]; APIGENIN [WHO-DD]; BiomolKI2_000082; 4,5, 7-Trihydroxyflavone; Lopac0_000065; Oprea1_622293; SCHEMBL19428; 4',5,7-trihydroxy-Flavone; Apigenin, analytical standard; BSPBio_000368; BSPBio_003384; KBioGR_002565; SPECTRUM200846; MLS000697626; MLS000859991; MLS001074874; MLS006011839; BIDD:ER0135; DivK1c_000798; SCHEMBL222227; SPBio_000416; SPBio_002307; ghl.PD_Mitscher_leg0.1194; BDBM7458; BPBio1_000406; GTPL4136; MEGxp0_000176; ACon1_002450; cid_5280443; HMS502H20; KBio1_000798; KBio3_002887; NINDS_000798; Bio1_000376; Bio1_000865; Bio1_001354; HMS1569C10; HMS1922P22; HMS2096C10; HMS2230D17; HMS3260M11; HMS3267D21; HMS3373B18; HMS3412A08; HMS3561P09; HMS3655D18; HMS3676A08; HMS3866D03; Apigenin, >=95.0% (HPLC); BCP28288; HY-N1201; Tox21_201542; Tox21_302884; Tox21_500065; Apigenin; 4',5,7-Trihydroxyflavone; BBL010499; CCG-40061; HB0117; HSCI1_000221; LMPK12110005; NSC815095; s2262; STK801630; ZB1873; AKOS002140699; AC-8011; CS-5432; DB07352; LP00065; ND-9076; NSC-815095; SDCCGMLS-0066379.P001; SDCCGSBI-0050053.P003; IDI1_000798; SMP2_000338; Apigenin, >=97% (TLC), from citrus; NCGC00015049-01; NCGC00015049-02; NCGC00015049-03; NCGC00015049-04; NCGC00015049-05; NCGC00015049-06; NCGC00015049-07; NCGC00015049-08; NCGC00015049-09; NCGC00015049-10; NCGC00015049-11; NCGC00015049-12; NCGC00015049-13; NCGC00015049-14; NCGC00015049-15; NCGC00015049-16; NCGC00015049-18; NCGC00015049-28; NCGC00025057-01; NCGC00025057-02; NCGC00025057-03; NCGC00025057-04; NCGC00025057-05; NCGC00025057-06; NCGC00025057-07; NCGC00025057-08; NCGC00025057-09; NCGC00169835-01; NCGC00169835-02; NCGC00169835-03; NCGC00256419-01; NCGC00259092-01; NCGC00260750-01; LY080400; NCI60_041830; SY005957; TS-00897; APIGENIN (CONSTITUENT OF CHAMOMILE); LY 080400; EU-0100065; FT-0622445; FT-0623582; FT-0662251; SW196866-2; A 3145; C01477; K00045; M01289; O11338; Apigenin 100 microg/mL in Acetonitrile:Methanol; Apigenin, >=97% (TLC), from parsley, powder; Biochem Biophys Res Comm 212: 767 (1997); EN300-7382221; 5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one; A828903; APIGENIN (CONSTITUENT OF CHAMOMILE) [DSC]; Apigenin, primary pharmaceutical reference standard; Q424567; 2-(P-HYDROXYPHENYL)-5,7-DIHYDROXY-CHROMONE; 4 inverted exclamation mark ,5,7-trihydroxyflavone; Q-100586; Q-200822; SR-01000075663-1; SR-01000075663-3; SR-01000075663-7; SR-01000075663-8; BRD-K01493881-001-10-4; BRD-K01493881-001-17-9; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #; Z1741982550; 4H-1-Benzopyran-4-one,7-dihydroxy-2-(4-hydroxyphenyl)-; D50A2D8A-6D8B-4708-B21E-2DE9580D033F; Apigenin, United States Pharmacopeia (USP) Reference Standard; 4H-1BENZOPYRAN-4-ONE,5,7-DIHYDROXY-2-(4-HYDROXY-PHENYL)-
Click to Show/Hide
|
||||
| Structure |
 |
||||
| Formula |
C15H10O5
|
||||
| IUPAC Name |
5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
||||
| Canonical SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O
|
||||
| InChI |
InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
|
||||
| InChIKey |
KZNIFHPLKGYRTM-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | Cellular tumor antigen p53 (TP53) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
5-weeks-old kainate (KA)-induced BALB/c nude mice, a widely used epilepsy mouse model, were performed with intraperitoneal (i.p.) injection of KA (6 mg/kg). Pre-treatment 21 with antioxidant apigenin (60 mg/Kg, 2 days) or post-treatment with apigenin (60 mg/Kg, 1 day), mice were injected with KA (6 mg/kg) via intraperitoneal (i.p.) injection, and then HCP (0.5 mg/Kg) were injected by intravenous (i.v.) injection. In vivo and Ex vivo fluorescence images of relative ClO levels in mice brains 5, 15, 30, 45, and 60 min post injection of HCP were further performed by using the IVIS Spectrum imaging system (Nanjing University) with an excitation filter of 430 nm and the collection wavelength range is from 500-600 nm.
Click to Show/Hide
|
||||
| Response regulation | Apigenin can efficiently reduce the expression of intracellular MPO and increase the levels of GPX4 and SIRT1, thereby conferring neuroprotection through regulation of kainic acid (KA)-induced ferroptosis. And the level of Ac-p53 inside the brains treated with apigenin was down-regulated, suggesting that the p53-mediated ferroptosis pathway might be blocked. Overall, apigenin was screened and confirmed as an efficient lead compound for epilepsy prevention and treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response regulation | DEHP caused oxidative stress and increased the Fe2+ content, finally resulting in ferroptosis in AML12 cells. Apigenin restrained the toxicity of DEHP and antagonized DEHP-induced ferroptosis in AML12 cells. The protective effects of APG on DEHP-induced liver injury were achieved by activating GPX4 and suppressing intracellular iron accumulation. | ||||
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
5-weeks-old kainate (KA)-induced BALB/c nude mice, a widely used epilepsy mouse model, were performed with intraperitoneal (i.p.) injection of KA (6 mg/kg). Pre-treatment 21 with antioxidant apigenin (60 mg/Kg, 2 days) or post-treatment with apigenin (60 mg/Kg, 1 day), mice were injected with KA (6 mg/kg) via intraperitoneal (i.p.) injection, and then HCP (0.5 mg/Kg) were injected by intravenous (i.v.) injection. In vivo and Ex vivo fluorescence images of relative ClO levels in mice brains 5, 15, 30, 45, and 60 min post injection of HCP were further performed by using the IVIS Spectrum imaging system (Nanjing University) with an excitation filter of 430 nm and the collection wavelength range is from 500-600 nm.
Click to Show/Hide
|
||||
| Response regulation | Apigenin can efficiently reduce the expression of intracellular MPO and increase the levels of GPX4 and SIRT1, thereby conferring neuroprotection through regulation of kainic acid (KA)-induced ferroptosis. And the level of Ac-p53 inside the brains treated with apigenin was down-regulated, suggesting that the p53-mediated ferroptosis pathway might be blocked. Overall, apigenin was screened and confirmed as an efficient lead compound for epilepsy prevention and treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Responsed Regulator | Amine oxidase [flavin-containing] B (MAOB) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HUVECs (Human umbilical vein endothelial cells) | ||||
| In Vivo Model |
Mice were exposed to 12/12 h of light/darkness and given free access to food and water. All C57BL/6J mice were fasted for 12 h and anesthetized by intraperitoneal injection of 1% sodium pentobarbital before modeling. Later, the superior mesenteric artery (SMA) was subjected to 45 min of global no-flow ischemia, followed by 90 min of reperfusion to induce IIRI. The successful model could be revealed by the microcirculation detector. In order to investigate the effects of APG, mice were randomly divided into different groups: Sham group, IIRI group, APG groups (2 mg/kg, 4 mg/kg and 8 mg/kg), ZnPPIX group (Protoporphyrin Zinc(), Dalian Meilun Biotech Co., Ltd., China, 10mg/kg), Selegiline group (10 mg/kg, Shandong Topscience Biotech Co., Ltd., China) and the ZnPPIX + Selegiline group (10 mg/kg ZnPPIX and 10 mg/kg Selegiline). As mentioned above, drug was intraperitoneal injected after a 10-min-ischemia.
Click to Show/Hide
|
||||
| Response regulation | MST analysis suggested that Apigenin could specifically bind to heme oxygenase 1 (HO-1) and monoamine oxidase b (MAO-B). Simultaneously, APG could attenuate ROS generation and Fe2+ accumulation, maintain mitochondria function thus inhibit ferroptosis and alleviate intestinal ischemia-reperfusion injury. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HUVECs (Human umbilical vein endothelial cells) | ||||
| In Vivo Model |
Mice were exposed to 12/12 h of light/darkness and given free access to food and water. All C57BL/6J mice were fasted for 12 h and anesthetized by intraperitoneal injection of 1% sodium pentobarbital before modeling. Later, the superior mesenteric artery (SMA) was subjected to 45 min of global no-flow ischemia, followed by 90 min of reperfusion to induce IIRI. The successful model could be revealed by the microcirculation detector. In order to investigate the effects of APG, mice were randomly divided into different groups: Sham group, IIRI group, APG groups (2 mg/kg, 4 mg/kg and 8 mg/kg), ZnPPIX group (Protoporphyrin Zinc(), Dalian Meilun Biotech Co., Ltd., China, 10mg/kg), Selegiline group (10 mg/kg, Shandong Topscience Biotech Co., Ltd., China) and the ZnPPIX + Selegiline group (10 mg/kg ZnPPIX and 10 mg/kg Selegiline). As mentioned above, drug was intraperitoneal injected after a 10-min-ischemia.
Click to Show/Hide
|
||||
| Response regulation | MST analysis suggested that Apigenin could specifically bind to heme oxygenase 1 (HO-1) and monoamine oxidase b (MAO-B). Simultaneously, APG could attenuate ROS generation and Fe2+ accumulation, maintain mitochondria function thus inhibit ferroptosis and alleviate intestinal ischemia-reperfusion injury. | ||||
References
