Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0083)
| Name |
Haloperidol
|
||||
|---|---|---|---|---|---|
| Synonyms |
haloperidol; 52-86-8; Haldol; Serenace; Aloperidin; Eukystol; Aloperidol; Brotopon; Serenase; Serenelfi; Linton; Dozic; Einalon S; Aloperidolo; Galoperidol; Halopoidol; Halojust; Halopal; Keselan; Mixidol; Ulcolind; Uliolind; Pernox; Sernas; Sernel; Aldo; Bioperidolo; Lealgin compositum; Sigaperidol; Peluces; Haldol Solutab; Aloperidon; Haloperidolum; Fortunan; Halosten; McN-JR-1625; 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one; Halol; Halidol; Halopidol; Neurodol; Pekuces; C21H23ClFNO2; R-1625; 4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-1-(4-fluorophenyl)butan-1-one; 177716-59-5; NSC 170973; R 1625; CCRIS 1630; 1-(3-p-Fluorobenzoylpropyl)-4-p-chlorophenyl-4-hydroxypiperidine; HSDB 3093; EINECS 200-155-6; 4'-Fluoro-4-(4-(p-chlorophenyl)-4-hydroxypiperidinyl)butyrophenone; 4'-Fluoro-4-(4-hydroxy-4-(4'-chlorophenyl)piperidino)butyrophenone; NSC 615296; NSC-170973; NSC-615296; UNII-J6292F8L3D; BRN 0331267; CHEBI:5613; Haloperidol (Haldol); DTXSID4034150; 4-(4-Hydroxy-4'-chloro-4-phenylpiperidino)-4'-fluorobutyrophenone; J6292F8L3D; 1-Butanone, 4-(4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)-; 4-(4-(4-Chlorophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)-1-butanone; CHEMBL54; NSC170973; Haldol (TN); 4-(4-(p-Chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone; Butyrophenone, 4'-fluoro-4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-; 4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone; MLS000028450; DTXCID2014150; 4'-Fluoro-4-(4-hydroxy-4-p-chlorophenylpiperidino)butyrophenone; 4-(4-(para-Chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone; 4-[4-(p-Chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone; gamma-(4-(p-Chlorophenyl)-4-hydroxypiperidino)-p-fluorbutyrophenone; gamma-(4-(p-Chlorophenyl)-4-hydroxypiperidino)-p-fluorobutyrophenone; 5-21-02-00377 (Beilstein Handbook Reference); Butyrophenone, 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluoro-; 1-Butanone, 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-; VESALIUM COMPONENT HALOPERIDOL; NSC615296; CAS-52-86-8; HALOPERIDOL COMPONENT OF VESALIUM; NCGC00015500-10; Aloperidolo [DCIT]; SMR000058303; Aloperidolo [Italian]; gamma-(4-(p-Chlorophenyl)-4-hydroxpiperidino)-p-fluorbutyrophenone; 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)-butan-1-one; HALOPERIDOL (MART.); HALOPERIDOL [MART.]; HALOPERIDOL (USP-RS); HALOPERIDOL [USP-RS]; Haloperidolum [INN-Latin]; Haloperido; HALOPERIDOL (EP MONOGRAPH); HALOPERIDOL (USP IMPURITY); HALOPERIDOL [EP MONOGRAPH]; HALOPERIDOL [USP IMPURITY]; Peridol; Dozix; HALOPERIDOL (USP MONOGRAPH); HALOPERIDOL [USP MONOGRAPH]; Apo-Haloperidol; Pms Haloperidol; Novo-Peridol; Butyrophenone, 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4'-fluoro-; Haldol La; SR-01000003076; Duraperidol; Epoxy resins; 4'-Fluoro-4-[4-hydroxy-4-(4'-chlorophenyl)piperidino]butyrophenone; gamma-[4-(p-chlorophenyl)-4-hydroxypiperidino]-p-fluorobutyrophenone; 4-[4-(para-Chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone; Picroside-III; Haloperidol, 1; 4-(4-(4-chlorophenyl)-4-hydroxy-1-piperidyl)-1-(4-fluorophenyl)-butan-1-one; Haloperidol, powder; Prestwick_250; Haloperidol [USAN:USP:INN:BAN:JAN]; PS14 - Haloperidol; Spectrum_000861; Tocris-0931; starbld0018801; Opera_ID_446; HALOPERIDOL [MI]; 4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-butan-1-one;propionate(HCl); 4-[4-(4-Chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone; Prestwick0_000115; Prestwick1_000115; Prestwick2_000115; Prestwick3_000115; Spectrum2_001268; Spectrum3_000448; Spectrum4_000570; Spectrum5_000788; HALOPERIDOL [INN]; HALOPERIDOL [JAN]; Lopac-H-1512; HALOPERIDOL [HSDB]; HALOPERIDOL [USAN]; Biomol-NT_000035; GTPL86; Probes1_000255; Probes2_000296; H 1512; HALOPERIDOL [VANDF]; SCHEMBL8264; Lopac0_000583; Oprea1_509923; BSPBio_000130; BSPBio_002096; HALOPERIDOL [WHO-DD]; HALOPERIDOL [WHO-IP]; KBioGR_000980; KBioGR_002390; KBioSS_001341; KBioSS_002395; MLS001146904; BIDD:GT0128; DivK1c_000654; SPECTRUM1500325; SPBio_001236; SPBio_002069; McM-JR-1625; BPBio1_000144; BPBio1_001231; Haloperidol (JP17/USP/INN); BDBM21398; Haloperidol, 1mg/ml in Methanol; HMS502A16; KBio1_000654; KBio2_001341; KBio2_002390; KBio2_003909; KBio2_004958; KBio2_006477; KBio2_007526; KBio3_001316; KBio3_002869; LNEPOXFFQSENCJ-UHFFFAOYSA-; HALOPERIDOL [ORANGE BOOK]; N05AD01; cMAP_000037; Haloperidol for system suitability; NINDS_000654; AC250; Haloperidol for peak identification; HMS1568G12; HMS1920D03; HMS2089M15; HMS2091J09; HMS2095G12; HMS2234P08; HMS3261F08; HMS3370H11; HMS3657I13; HMS3712G12; Pharmakon1600-01500325; BCP33202; CHA71659; Haloperidol 1.0 mg/ml in Methanol; STR04750; Tox21_110162; Tox21_300475; Tox21_500583; CCG-36042; CCG-39111; HALOPERIDOLUM [WHO-IP LATIN]; NSC757054; STL417208; 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)butan-1-one; AKOS000280660; Tox21_110162_1; AT13670; CS-1971; DB00502; LP00583; NSC-757054; SDCCGSBI-0050565.P005; IDI1_000654; MRF-0000027; QTL1_000042; WLN: T6NTJ A3VR DF& DQ DR DG; NCGC00015500-01; NCGC00015500-02; NCGC00015500-03; NCGC00015500-04; NCGC00015500-05; NCGC00015500-06; NCGC00015500-07; NCGC00015500-08; NCGC00015500-09; NCGC00015500-11; NCGC00015500-12; NCGC00015500-13; NCGC00015500-14; NCGC00015500-15; NCGC00015500-16; NCGC00015500-17; NCGC00015500-19; NCGC00015500-24; NCGC00015500-32; NCGC00016234-01; NCGC00023875-02; NCGC00023875-04; NCGC00023875-05; NCGC00023875-06; NCGC00023875-07; NCGC00023875-08; NCGC00023875-09; NCGC00254503-01; NCGC00261268-01; AC-19691; BH166165; HY-14538; SBI-0050565.P004; Haloperidol decanoate impurity, haloperidol-; SC 170973; AB00052008; EU-0100583; FT-0669100; FT-0669101; FT-0697842; H0912; S1920; SW196557-4; C01814; D00136; VU0239704-10; AB00052008-21; AB00052008-22; AB00052008_23; AB00052008_24; A899749; L000288; Q251347; SR-01000003076-2; SR-01000003076-8; W-105791; BRD-K67783091-001-04-8; BRD-K67783091-001-05-5; BRD-K67783091-003-03-6; HALOPERIDOL DECANOATE IMPURITY G [EP IMPURITY]; SR-01000003076-11; Z1590789254; Haloperidol, European Pharmacopoeia (EP) Reference Standard; .gamma.-[4-(p-Chlorphenyl)-4-hydroxpiperidino]-p-fluorbutyrophenone; HALOPERIDOL DECANOATE IMPURITY, HALOPERIDOL- [USP IMPURITY]; Haloperidol, United States Pharmacopeia (USP) Reference Standard; .gamma.-(4-(p-Chlorophenyl)-4-hydroxpiperidino)-p-fluorbutyrophenone; 4-(4-HYDROXY-4'-CHLORO-4-PHENYLPIPERIDINO)-4'-FLUORBUTYROPHENONE; Haloperidol, Pharmaceutical Secondary Standard; Certified Reference Material; Haloperidol for peak identification, European Pharmacopoeia (EP) Reference Standard; Haloperidol for system suitability, European Pharmacopoeia (EP) Reference Standard; InChI=1/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2
Click to Show/Hide
|
||||
| Structure |
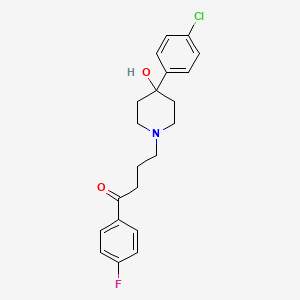 |
||||
| Formula |
C21H23ClFNO2
|
||||
| IUPAC Name |
4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one
|
||||
| Canonical SMILES |
C1CN(CCC1(C2=CC=C(C=C2)Cl)O)CCCC(=O)C3=CC=C(C=C3)F
|
||||
| InChI |
InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2
|
||||
| InChIKey |
LNEPOXFFQSENCJ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver/Suppressor | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response regulation | The study demonstrated that haloperidol strengthened sorafenib-induced ferroptosis in Hepatocellular carcinoma cell lines for the first time. During ferroptosis, haloperidol substantially increased the cellular levels of Fe2+, GSH and lipid peroxidation. Additionally, haloperidol induced the expression of HO-1. | |||
