Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10065)
| Target Name | Glutamate--cysteine ligase regulatory subunit (GCLM) | ||||
|---|---|---|---|---|---|
| Synonyms |
GLCLR; GCS light chain; Gamma-ECS regulatory subunit; Gamma-glutamylcysteine synthetase regulatory subunit; Glutamate--cysteine ligase modifier subunit
Click to Show/Hide
|
||||
| Gene Name | GCLM | ||||
| Sequence |
MGTDSRAAKALLARARTLHLQTGNLLNWGRLRKKCPSTHSEELHDCIQKTLNEWSSQINP
DLVREFPDVLECTVSHAVEKINPDEREEMKVSAKLFIVESNSSSSTRSAVDMACSVLGVA QLDSVIIASPPIEDGVNLSLEHLQPYWEELENLVQSKKIVAIGTSDLDKTQLEQLYQWAQ VKPNSNQVNLASCCVMPPDLTAFAKQFDIQLLTHNDPKELLSEASFQEALQESIPDIQAH EWVPLWLLRYSVIVKSRGIIKSKGYILQAKRRGS Click to Show/Hide
|
||||
| Family | Aldo/keto reductase family | ||||
| Function |
Sulfur metabolism; glutathione biosynthesis; glutathione from L-cysteine and L-glutamate: step 1/2.
Click to Show/Hide
|
||||
| Gene ID | 2730 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
GCLM can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Mitogen-activated protein kinase 8 (MAPK8)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | L-F001 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | L-F001 could restore GPX4 and glutamate-cysteine ligase modifier subunit (GCLM) levels, and significantly deceased Cyclooxygenase (COX-2) levels to rescue the lipid peroxidation imbalance. And L-F001 could reduce RSL3-induced c-Jun N-terminal kinase (JNK) activation, which might be a potential drug target for for the therapy of ferroptosis-related diseases, such as cerebral ischemia. | |||
hsa-miR-145-5p (miRNA)
Prolactinoma [ICD-11: 2F37]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Cabergoline | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
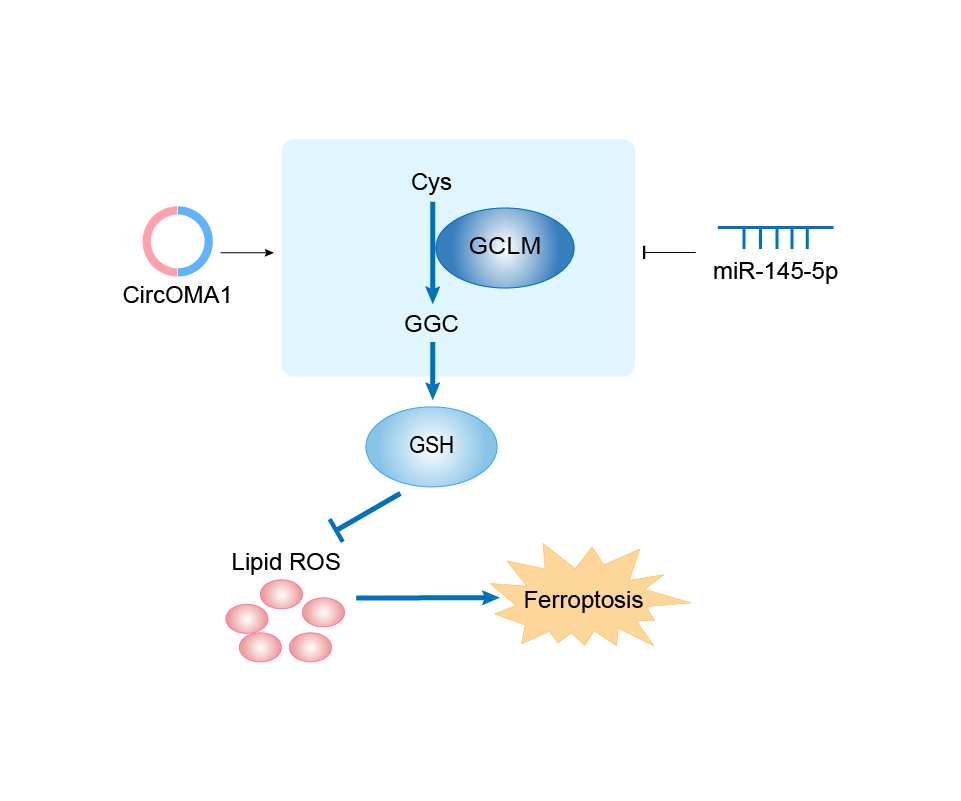
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
CircOMA1 (circRNA)
Prolactinoma [ICD-11: 2F37]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Cabergoline | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
Unspecific Regulator
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | |||
| Responsed Drug | Andrographis | Approved | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | |
| Response Description | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | |||
Mitogen-activated protein kinase 8 (MAPK8)
L-F001
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | L-F001 could restore GPX4 and glutamate-cysteine ligase modifier subunit (GCLM) levels, and significantly deceased Cyclooxygenase (COX-2) levels to rescue the lipid peroxidation imbalance. And L-F001 could reduce RSL3-induced c-Jun N-terminal kinase (JNK) activation, which might be a potential drug target for for the therapy of ferroptosis-related diseases, such as cerebral ischemia. | |||
hsa-miR-145-5p (miRNA)
Cabergoline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Prolactinoma [ICD-11: 2F37] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
CircOMA1 (circRNA)
Cabergoline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Prolactinoma [ICD-11: 2F37] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
Unspecific Regulator
Andrographis
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | |
| Response Description | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | |||
References
