Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0135)
| Name |
Decitabine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Decitabine; 5-Aza-2'-deoxycytidine; 2353-33-5; Dacogen; 2'-Deoxy-5-azacytidine; 5-Azadeoxycytidine; AzadC; 5-aza-CdR; 5-aza-dC; Dezocitidine; NSC 127716; Dac; 5-Aza-deoxycytidine; 4-amino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1,3,5-triazin-2(1H)-one; NSC-127716; 5A2dc; C8H12N4O4; 5-aza-2-deoxycytidine; 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one; MFCD00043011; 4-Amino-1-(2-deoxy-beta-D-erythro-pentofuranosyl)-s-triazin-2(1H)-one; 4-Amino-1-(2-deoxy-beta-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one; 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydro-1,3,5-triazin-2-one; MLS001332587; DTXSID7030432; CHEBI:50131; 776B62CQ27; SMR000857076; 2-Desoxy-5-azacytidine; 4-Amino-1-(2-deoxy-beta-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one; 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,3,5-triazin-2-one; 4-amino-1-[(2S,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one; Alpha-Decitibine; DTXCID5010432; 5-AZAdC; NSC127716; 2-deoxyazacytidine; Dacogen (TN); CAS-2353-33-5; NCGC_5ADOC; Decitabine (USAN/INN); Decitabine [USAN:INN:BAN]; CCRIS 8227; UNII-776B62CQ27; 5-Aza-2'-deoxycytidine;NSC 127716; NCGC00166088-01; Decitabine- Bio-X; deoxy-5-azacytidine; EINECS 219-089-4; BRN 0617982; Decitabine, Free Base; JNJ 30979754; JNJ-30979754; DECITABINE [MI]; DECITABINE [INN]; DECITABINE [USAN]; DECITABINE [VANDF]; MolMap_000063; E-7373; DECITABINE [MART.]; SCHEMBL4006; DECITABINE [WHO-DD]; MLS001332588; MLS006010136; ASTX-727; cid_451668; GTPL6805; CHEMBL1201129; DECITABINE [ORANGE BOOK]; BDBM96274; EX-A961; INQOVI COMPONENT DECITABINE; XAUDJQYHKZQPEU-KVQBGUIXSA-N; HMS2235O03; HMS3413L07; HMS3677L07; 105597-46-4; 22432-95-7; AMY33354; BCP02870; HY-A0004; 5-Aza-2'-deoxycytidine, >=97%; Tox21_112311; ASTX727 COMPONENT DECITABINE; HB1356; s1200; 5-aza-2'-deoxycytidine (Decitabine); DECITABINE COMPONENT OF INQOVI; 4-Amino-1-(2-deoxy-?-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one; AKOS015895047; ASTX-727 COMPONENT DECITABINE; Inqovi (decitabine + cedazuridine); Tox21_112311_1; AC-1135; BCP9000593; CCG-208143; CS-0372; DB01262; 1,3,5-Triazin-2(1H)-one, 4-amino-1-(2-deoxy-.beta.-D-erythro-pentofuranosyl)-; ASTX727 (decitabine + cedazuridine); Decitabine (NSC127716; 5AZA-CdR); NCGC00166088-02; NCGC00166088-05; AS-17558; BA164359; BCP0726000271; SW218076-2; D03665; EN300-269341; AB00918337-07; AB00918337_08; SR-01000838879; J-700084; Q1181878; SR-01000838879-4; Z2467077030; decitabine (2 inverted exclamation marka-deoxy-5-azacytidine).cd; s-Triazin-2(1H)-one, 4-amino-1-(2-deoxy-beta-D-erythro-pentofuranosyl)-; 1,3, 5-Triazin-2(1H)-one, 4-amino-1-(2-deoxy--D-erythro-pentofuranosyl)-; 4-AMINO-1-(2-DEOXY-.BETA.-D-ERYTHRO-PENTOFURANOSYL)-S-TRIAZIN-2(1H)-ONE; 4-Amino-1-(2-deoxy-beta-D-erythro-pento furanosyl)-1,3,5-triazin-2(1H)-one
Click to Show/Hide
|
||||
| Structure |
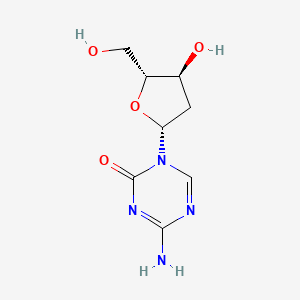 |
||||
| Formula |
C8H12N4O4
|
||||
| IUPAC Name |
4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one
|
||||
| Canonical SMILES |
C1C(C(OC1N2C=NC(=NC2=O)N)CO)O
|
||||
| InChI |
InChI=1S/C8H12N4O4/c9-7-10-3-12(8(15)11-7)6-1-4(14)5(2-13)16-6/h3-6,13-14H,1-2H2,(H2,9,11,15)/t4-,5+,6+/m0/s1
|
||||
| InChIKey |
XAUDJQYHKZQPEU-KVQBGUIXSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Myelodysplastic syndrome | ICD-11: 2A3Z | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell necroptosis | |||||
| In Vitro Model | SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | |
| MUTZ-1 cells | Burkitt lymphoma | Homo sapiens | CVCL_1431 | ||
| In Vivo Model |
C57BL/6 mice were purchased from Vital River (Beijing, China) at 6 to 8 weeks of age. Twenty mice were housed with five individuals per cage and used at a weight of approximately 20.0-22.0 g. They were randomly divided into four groups, five in each group, namely control group, low-dose group, middle-dose group, and high-dose group. The low-, middle-, and high-dose group mice were administered an intraperitoneal injection of 0.2-ml iron dextran at a concentration of 6.25, 12.5, and 25 mg/ml, respectively, every 3 days for 10 weeks to establish iron overload model. At the same time, normal saline was given to the control group.
Click to Show/Hide
|
||||
| Response regulation | Ferroptosis may account for the main mechanisms of how decitabine induced death of myelodysplastic syndrome (MDS) cells. Decitabine-induced ROS raise leads to ferroptosis in MDS cells by decreasing GSH level and GPX4 activity. | ||||
