Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0139)
| Name |
Paricalcitol
|
||||
|---|---|---|---|---|---|
| Synonyms |
Paricalcitol; 131918-61-1; Zemplar; Compound 49510; 19-Nor-1alpha,25-dihydroxyvitamin D2; Paracalcin; Compound-49510; (1R,3R)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]cyclohexane-1,3-diol; CHEBI:7931; DTXSID4048640; 6702D36OG5; (1R,3R,7E)-17beta-[(2R,3E,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-9,10-secoestra-5,7-diene-1,3-diol; NCGC00182706-01; Paricalcitol [USAN]; (1R,3R)-5-(2-((1R,3aS,7aR,E)-1-((2R,5S,E)-6-hydroxy-5,6-dimethylhept-3-en-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)cyclohexane-1,3-diol; 19-Nor-1,25-(OH)2D2; Zemplar (TN); 19-Nor-1-alpha,25-dihydroxyvitamin D2; HSDB 7360; Paricalcitol [USAN:USP:INN]; UNII-6702D36OG5; ABT-358; (7E,22E)-19-Nor-9,10-secoergosta-5,7,22-triene-1alpha,3beta,25-triol; PARICALCITOL [MI]; PARICALCITOL [JAN]; (1alpha.3beta,7E,22E)-19-Nor-9,10-secoergosta-5,7,22-triene-1,3,25-triol; PARICALCITOL [HSDB]; SCHEMBL3655; PARICALCITOL [VANDF]; PARICALCITOL [MART.]; BIDD:GT0330; PARICALCITOL [USP-RS]; PARICALCITOL [WHO-DD]; Paricalcitol (JAN/USP/INN); GTPL2791; CHEMBL1200622; DTXCID7028566; AMY2878; BPKAHTKRCLCHEA-UBFJEZKGSA-N; BDBM233195; PARICALCITOL [ORANGE BOOK]; PARICALCITOL [USP IMPURITY]; EX-A4434; PARICALCITOL [USP MONOGRAPH]; Tox21_112987; LMST04030163; s6681; AKOS005145562; BCP9001050; CS-0705; DB00910; 19-Nor-9,10-secoergosta-5,7,22-triene-1,3,25-triol, (1alpha,3beta,7E,22E)-; HY-50919; MS-27260; CAS-131918-61-1; C08127; D00930; A937163; Q155746; (1.ALPHA.3.BETA.,7E,22E)-19-NOR-9,10-SECOERGOSTA-5,7,22-TRIENE-1,3,25-TRIOL; (7E,22E)-19-NOR-9,10-SECOERGOSTA-5,7,22-TRIENE-1.ALPHA.,3.BETA.,25-TRIOL; (1R,3R,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-octahydro-1-[(1R,2E,4S)-5-hydroxy-1,4,5-trimethyl-2-hexen-1-yl]-7a-methyl-4H-inden-4-ylidene]ethylidene]-1,3-cyclohexanediol; 1,3-Cyclohexanediol, 5-[(2E)-2-[(1R,3aS,7aR)-octahydro-1-[(1R,2E,4S)-5-hydroxy-1,4,5-trimethyl-2-hexen-1-yl]-7a-methyl-4H-inden-4-ylidene]ethylidene]-, (1R,3R,5Z)-
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
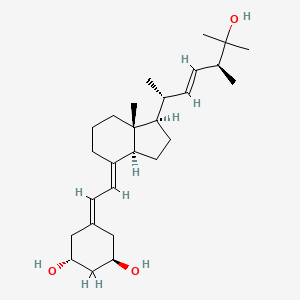 |
||||
| Formula |
C27H44O3
|
||||
| IUPAC Name |
(1R,3R)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]cyclohexane-1,3-diol
|
||||
| Canonical SMILES |
CC(C=CC(C)C(C)(C)O)C1CCC2C1(CCCC2=CC=C3CC(CC(C3)O)O)C
|
||||
| InChI |
InChI=1S/C27H44O3/c1-18(8-9-19(2)26(3,4)30)24-12-13-25-21(7-6-14-27(24,25)5)11-10-20-15-22(28)17-23(29)16-20/h8-11,18-19,22-25,28-30H,6-7,12-17H2,1-5H3/b9-8+,21-11+/t18-,19+,22-,23-,24-,25+,27-/m1/s1
|
||||
| InChIKey |
BPKAHTKRCLCHEA-UBFJEZKGSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute kidney failure | ICD-11: GB60 | |||
| Responsed Regulator | Vitamin D3 receptor (VDR) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
A total of 72 male C57BL/6 mice were purchased from Slyke jingda Biotechnology Company. They were randomly divided into five groups: Control group (n = 8), Cisplatin (20 mg/kg dissolved in saline) only group (n = 16), Cisplatin + paricalcitol (0.2 ug/kg dissolved in sterile water for injection and 20% propylene glycol) group (n = 16), Cisplatin + DMSO group (n = 16), Cisplatin + Fer-1 (5 mg/kg dissolved in DMSO) group (n = 16), were administered intraperitoneally. Cisplatin was injected once to mice, while Fer-1 was injected once an hour before cisplatin, and paricalcitol was injected once daily for five consecutive days before cisplatin. Each eight mice were sacrificed at 48 h and 72 h, respectively after cisplatin injection, and eight mice in the control group were sacrificed together with mice at 72 h.
Click to Show/Hide
|
||||
| Response regulation | Pretreatment of paricalcitol could also alleviated Erastin (an inducer of ferroptosis) induced cell death in HK-2 cell. Ferroptosis plays an important role in cisplatin induced acute kidney injury. VDR activation can protect against cisplatin induced renal injury by inhibiting ferroptosis partly via trans-regulation of GPX4. | ||||
