Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0159)
| Name |
Buthionine sulfoximine
|
||||
|---|---|---|---|---|---|
| Synonyms |
5072-26-4; BUTHIONINE SULFOXIMINE; Butionine sulfoximine; Buthionine sulphoximine; DL-Buthionine-[S,R]-sulfoximine; 2-Amino-4-(butylsulfonimidoyl)butanoic acid; Buthionine sulfoxamine; DL-Buthionine-(S,R)-sulfoximine; Butanoic acid, 2-amino-4-(S-butylsulfonimidoyl)-; 2-Amino-4-(S-butylsulfonimidoyl)butanoic acid; D,L-Buthionine-(S,R)-sulfoximine; DL-Buthionine-sulfoximine; Buthionine-S,R-sulfoximine; NSC 381100; dl-buthionine (s,r)-sulfoximine; 2-azaniumyl-4-(butylsulfonimidoyl)butanoate; S-butyl-DL-homocysteine (S,R)-sulfoximine; LW4108Q0BV; CHEBI:28714; DL-butathionine-(S,R)-sulfoximine; NSC326231; NSC381100; NSC-381100; S-Butyl-DL-homocysteine-[S,R]-sulfoximine; 2-amino-4-(S-butylsulfonimidoyl)butyric acid; BRN 2367136; Buthione sulfoximine; 71765-30-5; BSO; UNII-LW4108Q0BV; DL-Buthionine-S,R-sulfoximine; l-buthionine (s,r)-sulfoximine; Sulfoximine, S-(3-amino-3-carboxypropyl)-S-butyl-; MFCD00070309; Lopac0_000231; SCHEMBL62033; BSPBio_002464; SPECTRUM1505108; CHEMBL1256575; CHEMBL1627290; DTXSID6044434; CHEBI:176510; KJQFBVYMGADDTQ-UHFFFAOYSA-N; HMS3260P03; BUTHIONINE SULFOXIMINE [MI]; BCP24029; BCP27775; Tox21_500231; s2433; STL328818; AKOS022106364; DL-Buthionine-(S,R)-sulfoximine;BSO; BUTHIONINE SULFOXIMINE [WHO-DD]; CCG-204326; DB12870; LP00231; SDCCGSBI-0050219.P003; NCGC00015148-03; NCGC00015148-04; NCGC00015148-05; NCGC00015148-06; NCGC00015148-07; NCGC00015148-13; NCGC00093696-01; NCGC00093696-02; NCGC00093696-03; NCGC00260916-01; AS-67962; NCI60_002827; 2-Amino-4-(butylsulfonimidoyl)butanoicacid; HY-106376; CS-0025687; EU-0100231; FT-0624379; FT-0627738; FT-0663956; DL-Buthionine-sulfoximine, >=99.0% (TLC); 2-Amino-4-(butylsulfonimidoyl)butanoic acid #; B 2640; C04543; SR-01000075713; Q5002519; SR-01000075713-1; 2-amino-4-[butyl(imino)oxo-??-sulfanyl]butanoic acid; BRD-A04020513-001-01-9; D,L-Buthionine-(S,R)-sulfoximine (Butionine sulfoximine); dl-Buthionine(S,R)-sulfoximine (H-DL-Hcy(O,NH,Bu)-OH)
Click to Show/Hide
|
||||
| Structure |
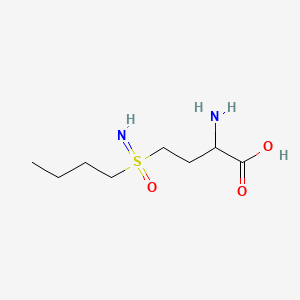 |
||||
| Formula |
C8H18N2O3S
|
||||
| IUPAC Name |
2-amino-4-(butylsulfonimidoyl)butanoic acid
|
||||
| Canonical SMILES |
CCCCS(=N)(=O)CCC(C(=O)O)N
|
||||
| InChI |
InChI=1S/C8H18N2O3S/c1-2-3-5-14(10,13)6-4-7(9)8(11)12/h7,10H,2-6,9H2,1H3,(H,11,12)
|
||||
| InChIKey |
KJQFBVYMGADDTQ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | VCaP cells | Prostate carcinoma | Homo sapiens | CVCL_2235 |
| LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| LNCaP C4-2 cells | Prostate carcinoma | Homo sapiens | CVCL_4782 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | |
| MDA-kb2 cells | Breast adenocarcinoma | Homo sapiens | CVCL_6421 | |
| Response regulation | ITC-ARi 13 and buthionine sulfoximine (BSO) cooperatively downregulate AR and induce ferroptosis likely through increasing the accessibility of 13/12b to cellular targets, escalating free intracellular ferrous iron and attenuating GSH-centered cellular defense and adaptation. Further studies on the combination of ITC-ARi and GSH synthesis inhibitor could result in a new modality against castration-resistant prostate cancer (CRPC). Collectively, the combination of ITC-ARi 13 and BSO reveals a pro-ferroptotic role of Nrf2 through upregulating HO-1 under GSH-deficient conditions. | |||
