Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0156)
| Name |
Perillaldehyde
|
||||
|---|---|---|---|---|---|
| Synonyms |
PERILLALDEHYDE; Perillyl aldehyde; 2111-75-3; Perilla aldehyde; Perillal; Perillic aldehyde; P-Mentha-1,8-dien-7-al; Dihydrocuminyl aldehyde; 4-prop-1-en-2-ylcyclohexene-1-carbaldehyde; 1-Cyclohexene-1-carboxaldehyde, 4-(1-methylethenyl)-; para-Mentha-1,8-dien-7-al; 4-Isopropenyl-1-cyclohexene-1-carboxaldehyde; FEMA No. 3557; Perillylaldehyde; 1-Cyclohexene-1-carboxaldehyde, 4-isopropenyl-; (+)-Perillaaldehyde; p-Mentha-1,8-dien-7-al (natural); 1,8-p-Menthadien-7-al; NSC 138642; 4-Isopropenylcyclohex-1-enecarbaldehyde; 6EQL0XA86G; CHEMBL469537; CHEBI:15421; DL-perillaldehyde(for perfumery); 4-(prop-1-en-2-yl)cyclohex-1-enecarbaldehyde; 4-(1-Methylethenyl)-1-cyclohexene-1-carboxaldehyde; 4-(prop-1-en-2-yl)cyclohex-1-ene-1-carbaldehyde; NSC-138642; 4-Isopropenyl-1-cyclohexene-1-carbaldehyde; (S)-(-)-Perillaldehyde; (S)-(-)-Perillic aldehyde; (S)-Perillaldehyde; l-Perillaldehyde; CCRIS 9128; dl-perillaldehyde; MFCD00001543; EINECS 218-302-8; UNII-6EQL0XA86G; PERILLALDEHYDE [MI]; PERILLALDEHYDE [INCI]; 1-Cyclohexene-1-carboxaldehyde, 4-(1-methylethenyl)-, (S)-; SCHEMBL221797; (+/-)-PERILLALDEHYDE; DTXSID6051855; BDBM50276351; MFCD00062990; NSC138642; AKOS015900800; LMPR0102090010; SY057816; P-MENTHA-1,8-DIEN-7-AL [FHFI]; 2-(4-Boc-piperazinyl)-4-phenylbutanoicacid; FT-0673634; FT-0686825; FT-0737059; P0866; 4-Isopropenyl-1-cyclohexene-1-carbaldehyde #; C02576; EN300-658599; 4-ISOPROPENYL-1-CYCLOHEXENECARBOXALDEHYDE; (-)-4-Isopropenyl-1-cyclohexene-1-carboxaldehyde; 4-(1-methylethenyl)-1-cyclohexene1-carboxyaldehyde; 4-(2-PROPENYL)-1-CYCLOHEXENECARBOXALDEHYDE; Q3117895; 0659C8EF-4608-42BD-9B0A-BB8B719E80F6; 4-(1-METHYLETHENYLPRO)-1-CYCLOHEXENE-1-CARBOXALDEHYDE
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
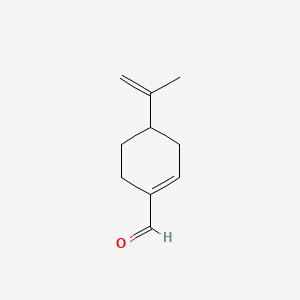 |
||||
| Formula |
C10H14O
|
||||
| IUPAC Name |
4-prop-1-en-2-ylcyclohexene-1-carbaldehyde
|
||||
| Canonical SMILES |
CC(=C)C1CCC(=CC1)C=O
|
||||
| InChI |
InChI=1S/C10H14O/c1-8(2)10-5-3-9(7-11)4-6-10/h3,7,10H,1,4-6H2,2H3
|
||||
| InChIKey |
RUMOYJJNUMEFDD-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Acute myeloid leukaemia | ICD-11: 2A60 | ||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Jurkat cells | T acute lymphoblastic leukemia | Homo sapiens | CVCL_0065 |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | |
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| Response regulation | We investigated and characterized its antileukemic potential in vitro, disclosing its ability to trigger ferroptosis. Specifically, perillaldehyde induced lipid peroxidation, decreased glutathione peroxidase 4 protein expression, and depleted intracellular glutathione on HL-60 promyelocytic leukemia cells. | |||
