Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0080)
| Name |
Olanzapine
|
||||
|---|---|---|---|---|---|
| Synonyms |
olanzapine; 132539-06-1; Zyprexa; Olansek; Zalasta; Zyprexa Zydis; Zyprexa Velotab; Zyprexa Intramuscular; Olanzapine Mylan; LY-170053; olanzapina; Zolafren; Zypadhera; Olazax; Olanzapine Teva; Olazax Disperzi; 2-Methyl-4-(4-methylpiperazin-1-yl)-10H-benzo[b]thieno[2,3-e][1,4]diazepine; Olanzapine Glenmark; olanzapinum; 2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3-b][1,5]benzodiazepine; LY 170053; Olzapin; Lanzac; Oferta; Olanzapine cipla; Olanzapine apotex; C17H20N4S; Olanzapine Neopharma; LY170053; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine; Olanzapin; Olanzapine Glenmark Europe; UNII-N7U69T4SZR; N7U69T4SZR; CHEBI:7735; DTXSID9023388; HSDB 8155; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno(2,3-b)(1,5)benzodiazepine; 2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno[3,2-c][1,5]benzodiazepine; 10H-thieno[2,3-b][1,5]benzodiazepine, 2-methyl-4-(4-methyl-1-piperazinyl)-; CHEMBL715; DTXCID503388; LYBALVI COMPONENT OLANZAPINE; SYMBYAX COMPONENT OLANZAPINE; 10H-Thieno(2,3-b)(1,5)benzodiazepine, 2-methyl-4-(4-methyl-1-piperazinyl)-; OLANZAPINE COMPONENT OF LYBALVI; OLANZAPINE COMPONENT OF SYMBYAX; 5-methyl-8-(4-methylpiperazin-1-yl)-4-thia-2,9-diazatricyclo[8.4.0.0^{3,7}]tetradeca-1(10),3(7),5,8,11,13-hexaene; NCGC00096077-03; OLANZAPINE (MART.); OLANZAPINE [MART.]; OLANZAPINE (USP-RS); OLANZAPINE [USP-RS]; OLANZAPINE (EP MONOGRAPH); OLANZAPINE [EP MONOGRAPH]; OLANZAPINE (USP MONOGRAPH); OLANZAPINE [USP MONOGRAPH]; Midax; Zydis; 2-methyl-4-(4-methyl-1-piperazinyl)-5H-thieno[3,2-c][1,5]benzodiazepine; Olanzapine Teva; SMR000466345; CAS-132539-06-1; SR-01000759343; ALKS-7921; 2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno(2,3-b)(1,5)benzodiazepine; Olanzapine [USAN:USP:INN:BAN]; Olanzapine; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine; LY 170053; Lanzac; Zyprexa; Olanzapine- Bio-X; MFCD00866702; Olanzapine (Zyprexa); KS-1090; PS15 - Olanzapine; OLANZAPINE [MI]; OLANZAPINE [INN]; OLANZAPINE [JAN]; OLANZAPINE [USAN]; OLANZAPINE [VANDF]; GTPL47; 2-Methyl-4-(4-methyl-1-piperazinyl)- 10H-thieno[2,3-b][1,5]benzodiazepine; OLANZAPINE [WHO-DD]; BIDD:PXR0138; SCHEMBL28763; Olanzapine (JAN/USP/INN); US8802672, Olanzapine; MLS000759457; MLS001165781; MLS001195646; MLS001424057; BIDD:GT0332; OLANZAPINE [EMA EPAR]; SCHEMBL117695; SPECTRUM1505024; Olanzapine (LY-170053); OLANZAPINE [ORANGE BOOK]; Olanzapine, >=98% (HPLC); BDBM35254; BDBM82479; CHEBI:94534; AMY7709; N05AH03; HMS2051H05; HMS2089M04; HMS2093I04; HMS2233F24; HMS3374L02; HMS3393H05; HMS3657I15; HMS3714J03; HMS3743A09; HMS3884J21; BCP04917; NSC_4585; Olanzapine, 1mg/ml in Acetonitrile; Tox21_111556; AC-665; HB1786; NSC754829; NSC801187; s2493; STK634338; STL388024; 2-methyl-4-(4-methylpiperazin-1-yl)-5H-thieno[2,3-b][1,5]benzodiazepine; AKOS000282888; AKOS005566122; Olanzapine 1.0 mg/ml in Acetonitrile; Tox21_111556_1; BCP9001021; CCG-100922; CS-1114; DB00334; NC00172; NSC-754829; NSC-801187; NCGC00096077-01; NCGC00096077-04; NCGC00096077-05; NCGC00096077-06; NCGC00096077-18; NCGC00389791-02; BO164166; HY-14541; SBI-0206786.P001; CAS_132539-06-1; FT-0673219; O0393; SW220248-1; C07322; D00454; EN300-119499; AB00639907-06; AB00639907-07; AB00639907_08; AB00639907_09; A806453; L000455; L005958; Q201872; J-006186; SR-01000759343-4; SR-01000759343-6; Z1521553472; Olanzapine, European Pharmacopoeia (EP) Reference Standard; Olanzapine, United States Pharmacopeia (USP) Reference Standard; Olanzapine, Pharmaceutical Secondary Standard; Certified Reference Material; (E)-2-methyl-4-(4-methylpiperazin-1-yl)-10H-benzo[b]thieno[2,3-e][1,4]diazepine; 2-Methyl-10-(4-methyl-piperazin-1-yl)-4H-3-thia-4,9-diaza-benzo[f]azulene; 2-methyl-4-(4-methyl-1-piperazinyl) -10h-thieno[2,3-b][1,5]benzodiazepine; 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno-[2,3-b][1,5]benzodiazepine; 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine #; 2-Methyl-4-(4-methylpiperazin-1-yl)-10H- thieno[2,3-b][1,5]benzodiazepin; Olanzapine for system suitability, European Pharmacopoeia (EP) Reference Standard; Olanzapine-d8, 100 mug/mL in acetonitrile, ampule of 1 mL, certified reference material; 5-methyl-8-(4-methylpiperazin-1-yl)-4-thia-2,9-diazatricyclo[8.4.0.0,3,7]tetradeca-1(10),3(7),5,8,11,13-hexaene
Click to Show/Hide
|
||||
| Structure |
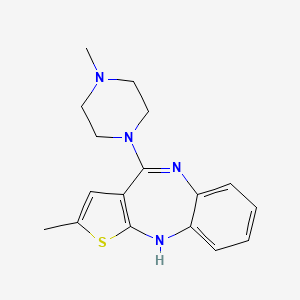 |
||||
| Formula |
C17H20N4S
|
||||
| IUPAC Name |
2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3-b][1,5]benzodiazepine
|
||||
| Canonical SMILES |
CC1=CC2=C(S1)NC3=CC=CC=C3N=C2N4CCN(CC4)C
|
||||
| InChI |
InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
|
||||
| InChIKey |
KVWDHTXUZHCGIO-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute pancreatitis | ICD-11: DC31 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | 266-6 cells | Normal | Mus musculus | CVCL_3481 | |
| In Vivo Model |
For cerulein-induced acute pancreatitis, male mice (age, 8-10 wk) received 7 hourly intraperitoneal injections of 50 g/kg cerulein in sterile saline. Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8-10 wk) were fed a LieberDeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington,NJ).In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the LieberDeCarli ethanol liquid diet.
Click to Show/Hide
|
||||
| Response regulation | Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice. | ||||
