Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0246)
| Name |
Wedelolactone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Wedelolactone; 524-12-9; 7-Methoxy-5,11,12-trihydroxycoumestan; 7-Methoxy-5,11,12-trihydroxy-coumestan; IKK Inhibitor II; 1,8,9-Trihydroxy-3-methoxycoumestan; CHEMBL97453; 0K6L725GNS; CHEBI:10037; 1,8,9-Trihydroxy-3-methoxy-6H-benzofuro[3,2-c]chromen-6-one; 5,11,12-Trihydroxy-7-methoxycoumestan; 1,8,9-trihydroxy-3-methoxy-[1]benzofuro[3,2-c]chromen-6-one; 6H-Benzofuro(3,2-c)(1)benzopyran-6-one, 1,8,9-trihydroxy-3-methoxy-; 1,8,9-Trihydroxy-3-methoxy-6H-[1]benzofuro[3,2-c]chromen-6-one; UNII-0K6L725GNS; 1,8,9-TRIHYDROXY-3-METHOXY-6H-(1)BENZOFURO(3,2-C)CHROMEN-6-ONE; MFCD07778564; 1,8,9-Trihydroxy-3-methoxy-6H-benzofuro[3,2-c][1]benzopyran-6-one; SCHEMBL601220; GTPL5551; DTXSID60200408; Wedelolactone, analytical standard; 1,8,9-trihydroxy-3-methoxy-benzofuro[3,2-c]chromen-6-one; HMS2043P19; BCP19859; HY-N0551; BDBM50096619; HB4124; LMPK12090046; s9042; AKOS015897173; CCG-208289; SMP2_000112; NCGC00163667-01; NCGC00163667-02; NCGC00163667-03; Wedelolactone, >=98% (HPLC), powder; AC-34562; CS-0009079; FT-0698529; W0015; A14804; K00058; A936784; SR-05000002318; Q6586709; SR-05000002318-2; BRD-K53635676-001-01-5; BRD-K53635676-001-02-3; Wedelolactone; 7-Methoxy-5,11,12-trihydroxy-coumestan; B0005-464576; 1,8,9-trihydroxy-3-methoxy-[1]benzoxolo[3,2-c]chromen-6-one; Wedelolactone, European Pharmacopoeia (EP) Reference Standard; 1,8,9-Trihydroxy-3-methoxy-benzo[4,5]furo[3,2-c]chromen-6-one
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
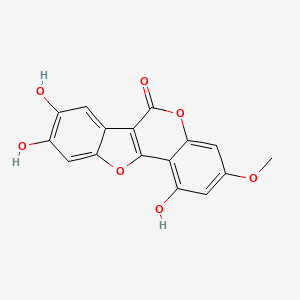 |
||||
| Formula |
C16H10O7
|
||||
| IUPAC Name |
1,8,9-trihydroxy-3-methoxy-[1]benzofuro[3,2-c]chromen-6-one
|
||||
| Canonical SMILES |
COC1=CC(=C2C(=C1)OC(=O)C3=C2OC4=CC(=C(C=C43)O)O)O
|
||||
| InChI |
InChI=1S/C16H10O7/c1-21-6-2-10(19)14-12(3-6)23-16(20)13-7-4-8(17)9(18)5-11(7)22-15(13)14/h2-5,17-19H,1H3
|
||||
| InChIKey |
XQDCKJKKMFWXGB-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute pancreatitis | ICD-11: DC31 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell pyroptosis | |||||
| In Vitro Model | AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
The 8-weeks old male Sprague-Dawley rats (bodyweight 250-300 g) were purchased from Liaoning changsheng biotechnology co. LTD (Benxi, China). The rats in the taurocholate-induced acute pancreatitis group (Taur, n = 6) were fasted overnight, after anesthesia the hepatic portal of the bile duct was clamped and 3.5% sodium taurocholate (Aladdin, Shanghai, China) in a volume of 1 ml/kg were retrogradely injected into the biliopancreatic duct at a constant speed (0.1 ml/min). The rats in the Sham group (n = 6) were received the laparotomy and the same volume of saline solution. The rats in the disulfiram treatment group (Taur + Disul, n = 6) were administrated with 100 mg/kg pyroptosis antagonist disulfiram (Aladdin) by intraperitoneal (i.p.) injection before the surgery. The rats in the ferrostatin-1 treatment group (Taur + Fer-1, n = 6) were i.p. administered 2.5 mol/kg ferroptosis antagonist ferrostatin-1 (Aladdin) 1 h before the surgery.
Click to Show/Hide
|
||||
| Response regulation | Wedelolactone promoted the transcriptional activity and the selenium sensitivity of GPX4. Moreover, the protective effects of Wed in caerulein-stimulated pancreatic acinar cells were markedly abrogated by the down-regulation of GPX4. Wed mitigated Acute pancreatitis (AP) and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. | ||||
