Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0291)
| Name |
Schisandrin B
|
||||
|---|---|---|---|---|---|
| Synonyms |
Schisandrin B; Schizandrin B; 61281-37-6; gamma-Schisandrin; CHEMBL479488; Schizandrin-B;Wuweizisu-B;gamma-Schisandrin; 3,4,5,19-tetramethoxy-9,10-dimethyl-15,17-dioxatetracyclo[10.7.0.02,7.014,18]nonadeca-1(19),2,4,6,12,14(18)-hexaene; S(-) Schisandrin B; Schizandrin-B; 1,2,3,13-tetramethoxy-6,7-dimethyl-5,6,7,8-tetrahydrobenzo[3',4']cycloocta[1',2':4,5]benzo[1,2-d][1,3]dioxole; ; A-Schisandrin; S(-) Wuweizisu B; Gamma-Schisandrin ,(S); SCHEMBL3380716; DTXSID60976772; GLXC-19128; HMS3652I13; BCP30085; HY-N0089; BDBM50341711; AKOS015897166; CCG-208607; CS-3659; NCGC00163663-01; FT-0775855; Schisandrin B; Wuweizisu-B; gamma-Schisandrin; SR-05000002175; Q-100707; SR-05000002175-2; (5S,6S,7S,13aS)-5,6,7,8-Tetrahydro-1,2,3,13-tetramethoxy-6,7-dimethylbenzo[3,4]cycloocta[1,2-f][1,3] benzodioxole-5,6-diol 5-Benzoate; 1,2,3,13-Tetramethoxy-6,7-dimethyl-5,6,7,8-tetrahydro-11H-benzo[3',4']cycloocta[1',2':4,5]benzo[1,2-d][1,3]dioxole; Benzo[3,4]cycloocta[1,2-f][1,3]benzodioxole,5,6,7,8-tetrahydro-1,2,3,13-tetramethoxy-6,7-dimethyl-, stereoisomer
Click to Show/Hide
|
||||
| Structure |
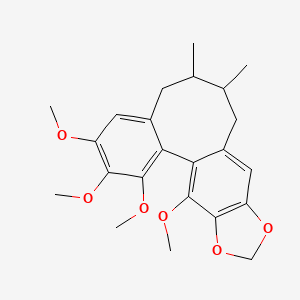 |
||||
| Formula |
C23H28O6
|
||||
| IUPAC Name |
3,4,5,19-tetramethoxy-9,10-dimethyl-15,17-dioxatetracyclo[10.7.0.02,7.014,18]nonadeca-1(19),2,4,6,12,14(18)-hexaene
|
||||
| Canonical SMILES |
CC1CC2=CC3=C(C(=C2C4=C(C(=C(C=C4CC1C)OC)OC)OC)OC)OCO3
|
||||
| InChI |
InChI=1S/C23H28O6/c1-12-7-14-9-16(24-3)20(25-4)22(26-5)18(14)19-15(8-13(12)2)10-17-21(23(19)27-6)29-11-28-17/h9-10,12-13H,7-8,11H2,1-6H3
|
||||
| InChIKey |
RTZKSTLPRTWFEV-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | rPHs (Rat primary hepatocytes) | ||||
| In Vivo Model |
A total of 40 male SD rats (180~200 g, 6~8 weeks) were purchased from the CMU experimental animal center. The rats were randomly divided into four groups: control (CON) group (normal diet rats were injected with equal volume of normal saline through caudal vein once a week, n = 10), SchB group (SchB diet rats, 50 mg/kg/day, were injected with equal volume of normal saline through caudal vein once a week, n = 10), THP group (normal diet rats were injected with 3 mg/kg/day THP through caudal vein once a week, n = 10), and SchB+THP group (SchB diet rats, 50 mg/kg/day, were injected with 3 mg/kg/day THP through caudal vein once a week, n = 10). CON and THP rats were fed an AIN-76A feed (12.4% fat, 68.8% carbohydrate, and 18.8% protein). SchB and SchB+THP rats were fed an SchB feed (approximately 0.5 SchB was added into AIN-76A feed). After conversion, 0.5 SchB in feed = 50 mg/kg in rats.
Click to Show/Hide
|
||||
| Response regulation | Schisandrin B (SchB) increased the levels of SOD, GSH, GSH-px, CAT, and T-AOC, decreased the level of MDA, and inhibited the abnormal oxidative stress in the liver. And SchB as a natural molecule depends on reducing the level of oxidative stress, thereby inhibiting lipid peroxidation, ferroptosis, and apoptosis. The expression of NRF2, GPX4, SOD2, and Bcl-2/Bax decreased, while the expression of NOX2/4 and cleaved caspase-3 increased in pirarubicin-treated hepatocytes. However, the above changes were significantly reversed after SchB or Fer-1 treatment. SchB has obvious protective effect on pirarubicin-induced hepatotoxicity. | ||||
