Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0254)
| Name |
Icariside II
|
||||
|---|---|---|---|---|---|
| Synonyms |
Baohuoside I; 113558-15-9; Icariside II; Icarlin II; BAOHUOSIDEI; CHEBI:82619; 5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one; CHEMBL560116; 3,5,7-Trihydroxy-4'-methoxyl-8-prenylflavone-3-O-rhamnopyranoside; Icariin-II;Icariside-II; Baohuoside-I; Icariside-II; Icariin-II; AMY496; SCHEMBL4229321; GTPL10686; DTXSID40150457; 2h44; EX-A6795; HY-N0011; BDBM50503751; AKOS037514560; CS-3673; 4H-1-Benzopyran-4-one, 3-((6-deoxy-alpha-L-mannopyranosyl)oxy)-5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-butenyl)-; AC-33977; AS-75062; anhydroicaritin-3-O-alpha-L-rhamnopyranoside; Baohuoside I; Anhydroicaritin; Icariside II; A894345; Q-100071; Q27156136; 3,5,7-trihydroxy-4'-methoxy-8-prenylflavone-3-O-rhamnopyranoside; 3,5,7-trihydroxy-4'-methoxy-8-prenylflavone-3-O-alpha-L-rhamnopyranoside; 3,5,7-Trihydroxy-4'-methoxyl-8-prenylflavone-3-O-rhamnopyranoside; 4H-1-Benzopyran-4-one, 3-((6-deoxy-alpha-L-mannopyranosyl)oxy)-5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-butenyl)-; 5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-4-oxo-4H-chromen-3-yl 6-deoxy-alpha-L-mannopyranosideohuside I; Icariin II; Icariside II; 4H-1-Benzopyran-4-one, 3-[(6-deoxy-alpha-L-mannopyranosyl)oxy]-5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-; 5,7-Dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one; 5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-chromen-4-one; 5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-4-oxo-4H-chromen-3-yl 6-deoxy-alpha-L-mannopyranoside
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
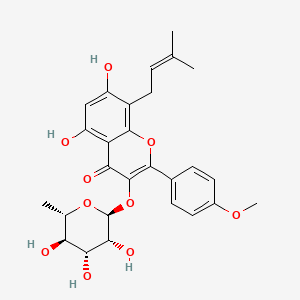 |
||||
| Formula |
C27H30O10
|
||||
| IUPAC Name |
5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one
|
||||
| Canonical SMILES |
CC1C(C(C(C(O1)OC2=C(OC3=C(C(=CC(=C3C2=O)O)O)CC=C(C)C)C4=CC=C(C=C4)OC)O)O)O
|
||||
| InChI |
InChI=1S/C27H30O10/c1-12(2)5-10-16-17(28)11-18(29)19-21(31)26(37-27-23(33)22(32)20(30)13(3)35-27)24(36-25(16)19)14-6-8-15(34-4)9-7-14/h5-9,11,13,20,22-23,27-30,32-33H,10H2,1-4H3/t13-,20-,22+,23+,27-/m0/s1
|
||||
| InChIKey |
NGMYNFJANBHLKA-LVKFHIPRSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hereditary Leiomyomatosis | ICD-11: 2C90 | |||
| Responsed Regulator | hsa-miR-324-3p (miRNA) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
A total of 30 male BALB/c nude mice (4-6 weeks old; 18-23 g) were randomized into four groups (7-8 mice per group): i) control group; ii) treated with 15 mg/kg ICS II; iii) treated with 25 mg/kg ICS II; and, iv) treated with 35 mg/kg ICS II. ACHN and Caki-1 cells (1 x 107) were suspended in 50 ul MEM media mixed with 50 ul Matrigel (BD Biosciences) and injected subcutaneously into the right flank of mice with 1.5%pentobarbital sodium (60 mg/kg body weight; intraperitoneal injection) under anesthesia. Weight lossof more than 20% was considered a humane endpoint.
Click to Show/Hide
|
||||
| Response regulation | Icariside II (ICS II) treatment triggered ferroptosis in renal cell carcinoma (RCC) cells by downregulating GPX4 in a p53-independent manner. Furthermore, ICS II treatment resulted in upregulation of miR-324-3p, which negatively regulated the expression of GPX4. | ||||
