Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0277)
| Name |
l-Buthionine sulfoximine
|
||||
|---|---|---|---|---|---|
| Synonyms |
l-buthionine sulfoximine; 83730-53-4; L-Buthionine-(S,R)-sulfoximine; L-Buthionine-sulfoximine; (2S)-2-Amino-4-(butylsulfonimidoyl)butanoic acid; Butanoic acid, 2-amino-4-(S-butylsulfonimidoyl)-, (2S)-; Buthionine sulfoximine, L-; (2S)-2-Amino-4-(S-butylsulfonimidoyl)butanoic Acid; BUTHIONINE SULFOXIME; NSC 326231; EEY8DZS103; NSC-326231; L-BSO; BRN 2367136; l-buthionine(s,r)-sulfoximine; 2-amino-4-(S-butylsulfonimidoyl)butanoate; UNII-EEY8DZS103; L-Buthionine (SR)-sulfoximine; Buthionine-S,R-sulfoximine, L-; L-Buthionine-(S,R)-sulphoximine; MFCD00067000; L-Butionine sulfoximine; NCIMech_000342; BUTHIONINE-SULFOXIMINE; Lopac0_000221; SCHEMBL62034; CHEMBL261642; l-buthionine-(r,s)-sulfoximine; CHEBI:94288; DTXSID70894150; HMS3260N03; BSO; Tox21_500221; BDBM50487312; NSC801426; s9728; AKOS027320570; CS-W020947; HY-106376A; LP00221; NSC-801426; SDCCGSBI-0050209.P002; BSO (L-Buthionine-(S,R)-sulfoximine); NCGC00093690-01; NCGC00093690-02; NCGC00093690-03; NCGC00093690-11; NCGC00260906-01; AS-69850; L-Buthionine-sulfoximine, >=97% (TLC); EU-0100221; B 2515; A857917; L-Buthionine-sulfoximine (H-L-Hcy(O,NH,Bu)-OH); SR-01000075712; SR-01000075712-1; BRD-A47706533-001-01-8; Q27166100; (2S)-2-AMINO-4-[BUTYL(IMINO)OXO-??-SULFANYL]BUTANOIC ACID; Butanoic acid, 2-amino-4-((R)-S-butylsulfonimidoyl)-, (2S)-rel-
Click to Show/Hide
|
||||
| Structure |
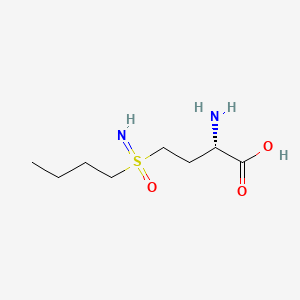 |
||||
| Formula |
C8H18N2O3S
|
||||
| IUPAC Name |
(2S)-2-amino-4-(butylsulfonimidoyl)butanoic acid
|
||||
| Canonical SMILES |
CCCCS(=N)(=O)CCC(C(=O)O)N
|
||||
| InChI |
InChI=1S/C8H18N2O3S/c1-2-3-5-14(10,13)6-4-7(9)8(11)12/h7,10H,2-6,9H2,1H3,(H,11,12)/t7-,14?/m0/s1
|
||||
| InChIKey |
KJQFBVYMGADDTQ-CVSPRKDYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response regulation | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | |||
