Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0012)
| Name |
Palmitic acid
|
||||
|---|---|---|---|---|---|
| Synonyms |
palmitic acid; Hexadecanoic acid; 57-10-3; Cetylic acid; palmitate; n-Hexadecanoic acid; Hexadecylic acid; Hydrofol; n-Hexadecoic acid; 1-Pentadecanecarboxylic acid; Palmitinic acid; hexaectylic acid; Pentadecanecarboxylic acid; hexadecoic acid; 1-Hexyldecanoic Acid; Industrene 4516; Emersol 140; Emersol 143; Hystrene 8016; Hystrene 9016; Palmitinsaeure; Palmitic acid, pure; Palmitic acid 95%; Kortacid 1698; FEMA No. 2832; Loxiol EP 278; Palmitic acid (natural); Hydrofol Acid 1690; Cetyl acid; Prifac 2960; C16:0; HSDB 5001; Pristerene 4934; Pristerene-4934; Edenor C16; NSC 5030; AI3-01594; Lunac P 95KC; Lunac P 95; Lunac P 98; CCRIS 5443; Prifac-2960; CHEBI:15756; NSC5030; NSC-5030; EINECS 200-312-9; UNII-2V16EO95H1; FA 16:0; BRN 0607489; Palmitic acid (NF); DTXSID2021602; Glycon P-45; IMEX C 1498; 2V16EO95H1; Hexadecanoic acid (9CI); MFCD00002747; Palmitic acid (7CI,8CI); CHEMBL82293; DTXCID101602; CH3-[CH2]14-COOH; EC 200-312-9; 4-02-00-01157 (Beilstein Handbook Reference); n-hexadecoate; LMFA01010001; PA 900; EDENOR C 16-98-100; 67701-02-4; FA 1695; SURFAXIN COMPONENT PALMITIC ACID; 1-hexyldecanoate; NCGC00164358-01; LUCINACTANT COMPONENT PALMITIC ACID; pentadecanecarboxylate; Hexadecanoic acid 10 microg/mL in Acetonitrile; HEXADECANOIC-11,11,12,12-D4 ACID; PALMITIC ACID (II); PALMITIC ACID [II]; PALMITIC ACID (MART.); PALMITIC ACID [MART.]; CH3-(CH2)14-COOH; Palmitic acid; Hexadecanoic acid; PLM; palmic acid; Hexadecanoate (n-C16:0); PALMITIC ACID (EP MONOGRAPH); PALMITIC ACID [EP MONOGRAPH]; Acid, Palmitic; CAS-57-10-3; Acid, Hexadecanoic; SR-01000944716; Palmitic acid [USAN:NF]; palmitoate; Hexadecoate; Palmitinate; Palmitinsaure; palmitic-acid; palmitoic acid; Hexadecanoicacid; Aethalic acid; Hexadecanoic acid Palmitic acid; 2hmb; 2hnx; Palmitic acid_jeyam; n-Hexadecyclic Acid; fatty acid 16:0; Palmitic Acid, FCC; Kortacid 1695; Palmitic acid_RaGuSa; Univol U332; 1219802-61-5; Prifrac 2960; Hexadecanoic acid anion; Hexadecanoic--d5 Acid; 3v2q; Palmitic acid, >=99%; bmse000590; Epitope ID:141181; CETYL ACID [VANDF]; PALMITIC ACID [MI]; SCHEMBL6177; PALMITIC ACID [DSC]; PALMITIC ACID [FCC]; PALMITIC ACID [FHFI]; PALMITIC ACID [HSDB]; PALMITIC ACID [INCI]; PALMITIC ACID [USAN]; FAT; WLN: QV15; P5585_SIGMA; PALMITIC ACID [VANDF]; GTPL1055; QSPL 166; PALMITIC ACID [USP-RS]; PALMITIC ACID [WHO-DD]; (1(1)(3)C)hexadecanoic acid; 1b56; HMS3649N08; Palmitic acid, analytical standard; Palmitic acid, BioXtra, >=99%; Palmitic acid, Grade II, ~95%; HY-N0830; Palmitic acid, natural, 98%, FG; Tox21_112105; Tox21_201671; Tox21_302966; AC9381; BBL011563; BDBM50152850; s3794; STL146733; Palmitic acid, >=95%, FCC, FG; AKOS005720983; Tox21_112105_1; CCG-267027; CR-0047; DB03796; Palmitic acid, for synthesis, 98.0%; NCGC00164358-02; NCGC00164358-03; NCGC00256424-01; NCGC00259220-01; BP-27917; Palmitic acid, purum, >=98.0% (GC); SY006518; CS-0009861; FT-0626965; FT-0772579; P0002; P1145; Palmitic acid, SAJ first grade, >=95.0%; EN300-19603; A14813; C00249; D05341; Palmitic acid, Vetec(TM) reagent grade, 98%; PALMITIC ACID (CONSTITUENT OF SPIRULINA); Palmitic acid, >=98% palmitic acid basis (GC); A831313; Q209727; PALMITIC ACID (CONSTITUENT OF FLAX SEED OIL); PALMITIC ACID (CONSTITUENT OF SAW PALMETTO); SR-01000944716-1; SR-01000944716-2; BA71C79B-C9B1-451A-A5BE-B480B5CC7D0C; PALMITIC ACID (CONSTITUENT OF BORAGE SEED OIL); PALMITIC ACID (CONSTITUENT OF SPIRULINA) [DSC]; F0001-1488; Z104474418; PALMITIC ACID (CONSTITUENT OF EVENING PRIMROSE OIL); PALMITIC ACID (CONSTITUENT OF SAW PALMETTO) [DSC]; Palmitic acid, certified reference material, TraceCERT(R); Palmitic acid, European Pharmacopoeia (EP) Reference Standard; Palmitic acid, United States Pharmacopeia (USP) Reference Standard; Palmitic acid, Pharmaceutical Secondary Standard; Certified Reference Material; Sodium Palmitate, Palmitic acid sodium salt, Sodium hexadecanoate, Sodium pentadecanecarboxylate, HSDB 759
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
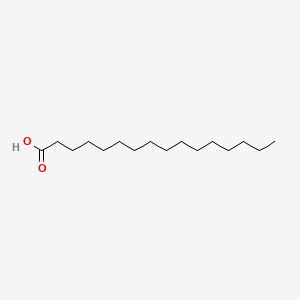 |
||||
| Formula |
C16H32O2
|
||||
| IUPAC Name |
hexadecanoic acid
|
||||
| Canonical SMILES |
CCCCCCCCCCCCCCCC(=O)O
|
||||
| InChI |
InChI=1S/C16H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18/h2-15H2,1H3,(H,17,18)
|
||||
| InChIKey |
IPCSVZSSVZVIGE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Health | ICD-11: N.A. | |||
| Responsed Regulator | Heat shock factor protein 1 (HSF1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Hsf1 and Hsf1+/+-/- mice were kindly given as a present by Dr. Ivor J. Benjamin (Froedtert & Medical College of Wisconsin, Milwaukee, WI, USA). Sex-matched Hsf1-/- mice and Hsf1 littermates were used at 16-20 weeks old. Each mouse was injected intraperitoneally with 2.5 umol PA (dissolved in 0.5 mL 10% BSA) or an equal volume of BSA twice daily for 7 days.
Click to Show/Hide
|
||||
| Response regulation | Palmitic acid (PA) decreased the protein expression levels of both heat shock factor 1 (HSF1) and glutathione peroxidase 4 (GPX4) in a dose- and time-dependent manner, which were restored by different ferroptosis inhibitors. Altogether, HSF1 may function as a key defender against PA-induced ferroptosis in cardiomyocytes by maintaining cellular iron homeostasis and GPX4 expression. | ||||
