Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0016)
| Name |
Disulfiram
|
||||
|---|---|---|---|---|---|
| Synonyms |
disulfiram; Tetraethylthiuram disulfide; 97-77-8; Antabuse; Bis(diethylthiocarbamoyl) disulfide; Antabus; TETD; Alcophobin; Anticol; Esperal; Teturam; Dicupral; Exhorran; Hoca; Ethyldithiurame; Abstensil; Antaethyl; Antietanol; Antivitium; Contralin; Tetradine; Tetraetil; Teturamin; Abstinil; Abstinyl; Antadix; Antalcol; Antetan; Antetil; Antietil; Antikol; Aversan; Averzan; Cronetal; Krotenal; Refusal; Etabus; Ethyl tuads; Ethyl Thiram; Ethyl Thiurad; Ethyl Tuex; Antaenyl; Antaetil; Antiaethan; Contrapot; Disulfan; Disulfuram; Ephorran; Stopetyl; Thiuranide; Anteyl; Bonibal; Disetil; Nocbin; Tenurid; Tenutex; Tetidis; Ekagom TEDS; Ekagom TETDS; Ethyldithiourame; Noxal; Anti-ethyl; Alk-aubs; Tetraethylthiuram disulphide; Thiuram E; TATD; Soxinol TET; Tetraethylthiram disulfide; Ekagom DTET; Accel TET; Espenal; Exhoran; Sanceler TET-G; Ro-sulfiram; Tetraethylthiuram; Tuads, ethyl; Usaf B-33; Sanceler TET; Tetraethylthioperoxydicarbonic diamide; Stopaethyl; Thireranide; Antaethan; Antethyl; Tetradin; Tillram; Accel TET-R; Ethyl Thiudad; Dupon 4472; Tetraethylthiuran disulfide; Nocceler TET; Nocceler TET-G; Anthethyl; Disulphuram; Stopethyl; Dupont fungicide 4472; Hocakrotenalnci-C02959; Tetraethylthiram disulphide; Bis(diethylthiocarbamyl) disulfide; Stopety; THIOCID; Ancazide ET; Etyl Tuex; Tetraethylthiuram sulfide; Thiuram disulfide, tetraethyl-; Akrochem TETD; Perkacit TETD; Ekaland TETD; Perkait TETD; N,N,N',N'-Tetraethylthiuram disulfide; Antabuse (TN); Bis(N,N-diethylthiocarbamoyl) disulfide; Ethyl Tuads Rodform; C10H20N2S4; 1,1'-Dithiobis(N,N-diethylthioformamide); Disulfide, bis(diethylthiocarbamoyl); ENT 27,340; NSC 190940; Thioperoxydicarbonic diamide, tetraethyl-; Bis(diethylthiocarbamoyl)disulphide; NCI-C02959; diethylcarbamothioylsulfanyl N,N-diethylcarbamodithioate; NSC-25953; N,N-diethyl[(diethylcarbamothioyl)disulfanyl]carbothioamide; Bis(N,N-diethylthiocarbamoyl)disulphide; N,N,N',N'-Tetraethylthiuram disulphide; Bis((diethylamino)thioxomethyl)disulphide; Bis((diethylamino)thioxomethyl) disulfide; Tetraethylthiuram disulfide;TETD; TTS; Disulfiram (Antabuse); TR3MLJ1UAI; Thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), tetraethyl-; CHEMBL964; MLS000069818; CHEBI:4659; ORA102; DTXSID1021322; ORA-102; Bis(diethylthiocarbamyoyl)disulfide; NSC25953; Esperal [France]; CAS-97-77-8; NCGC00016000-08; NCGC00016000-13; SMR000059171; Thioperoxydicarbonic diamide (((H2N)C(S))2S2), tetraethyl-; 1,1',1'',1'''-[disulfanediylbis(carbonothioylnitrilo)]tetraethane; DTXCID101322; Gababentin; Disulfram; Disulfiramum [INN-Latin]; Disulfiramo [INN-Spanish]; Bis[(diethylamino)thioxomethyl] disulfide; CCRIS 582; 1,1'-Dithiobis[N,N-diethylthioformamide]; TTS x; HSDB 3317; SR-01000076145; UNII-TR3MLJ1UAI; EINECS 202-607-8; MFCD00009048; NSC 25953; AI3-27340; Formamide, 1,1'-dithiobis(N,N-diethylthio-; Thioperoxydicarbonic diamide (((H2N)C(S))2S2), N,N,N',N'-tetraethyl-; Thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), N,N,N',N'-tetraethyl-; Disulfiram [USP:INN:BAN:JAN]; Prestwick_182; Spectrum_001010; CPD000059171; DISULFIRAM [MI]; Opera_ID_224; DISULFIRAM [INN]; DISULFIRAM [JAN]; Prestwick0_000097; Prestwick1_000097; Prestwick2_000097; Prestwick3_000097; Spectrum2_001176; Spectrum3_000405; Spectrum4_000228; Spectrum5_001590; DISULFIRAM [HSDB]; DISULFIRAM [IARC]; Lopac-T-1132; 1,N-diethylthioformamide]; DISULFIRAM [VANDF]; Formamide, 1,1'-dithiobis(N,N-diethylthio)-; UPCMLD-DP090; EC 202-607-8; T 1132; tetraethyl thiuram disulfide; DISULFIRAM [MART.]; Tetraethyldithiuram disulfide; DISULFIRAM [WHO-DD]; Lopac0_001164; SCHEMBL27213; BSPBio_000054; BSPBio_001930; KBioGR_000895; KBioSS_001490; MLS000758264; MLS001076475; MLS001423963; SPECTRUM1500262; SPBio_001191; SPBio_001993; BPBio1_000060; Disulfiram (JP17/USP/INN); GTPL7168; DISULFIRAM [EP IMPURITY]; DISULFIRAM [ORANGE BOOK]; UPCMLD-DP090:001; KBio2_001490; KBio2_004058; KBio2_006626; KBio3_001150; DISULFIRAM [EP MONOGRAPH]; Thioperoxydicarbonic diamide ((H2N)C(S))2S2, tetraethyl-; DISULFIRAM [USP MONOGRAPH]; HMS1568C16; HMS1920I16; HMS2051M17; HMS2090C18; HMS2091O22; HMS2095C16; HMS2230K06; HMS3263J09; HMS3371B21; HMS3393M17; HMS3655I19; HMS3712C16; HMS3867H13; Pharmakon1600-01500262; BCP07331; HY-B0240; bis-(diethyl-thiocarbamyl)-disulfide; Tox21_110280; Tox21_300403; Tox21_400072; Tox21_501164; BDBM50058655; CCG-39549; DL-379; HB1119; N,N',N'-Tetraethylthiuram disulfide; NSC756748; NSC800739; s1680; STL069539; 1,1',1'',1'''-{disulfanediylbis[(thioxomethylene)-nitrilo]}tetraethane; AKOS000120201; Tox21_110280_1; AT13284; DB00822; HS-0057; LP01164; NC00063; NSC-756748; NSC-800739; SDCCGSBI-0051131.P005; WLN: 2N2 & YUS & S 2; NCGC00016000-01; NCGC00016000-02; NCGC00016000-03; NCGC00016000-04; NCGC00016000-05; NCGC00016000-06; NCGC00016000-07; NCGC00016000-09; NCGC00016000-10; NCGC00016000-11; NCGC00016000-12; NCGC00016000-14; NCGC00016000-15; NCGC00016000-18; NCGC00016000-29; NCGC00094423-01; NCGC00094423-02; NCGC00094423-03; NCGC00094423-05; NCGC00094423-06; NCGC00094423-07; NCGC00254447-01; NCGC00261849-01; N,N,N'',N''-tetraethylthiuram disulfide; BIS(DIETHYLTHIOCARBAMOYL) DISULPHIDE; Formamide,1'-dithiobis(N,N-diethylthio)-; SBI-0051131.P004; 1,1''-dithiobis(N,N-diethylthioformamide); AB00051976; B0479; EU-0101164; FT-0631502; FT-0667720; SW196492-4; Tetraethylthiuram disulfide, >=97.0% (S); EN300-19458; C01692; D00131; S00294; AB00051976-20; AB00051976-21; AB00051976-23; AB00051976_22; AB00051976_25; A845750; Q409665; Q-201812; SR-01000076145-1; SR-01000076145-5; SR-01000076145-8; BRD-K32744045-001-05-6; BRD-K32744045-001-17-1; Z104473910; Disulfiram, British Pharmacopoeia (BP) Reference Standard; Disulfiram, European Pharmacopoeia (EP) Reference Standard; Disulfiram, United States Pharmacopeia (USP) Reference Standard; 1,1'',1'''',1''''''-[disulfanediylbis(carbonothioylnitrilo)]tetraethane; Disulfiram, Pharmaceutical Secondary Standard; Certified Reference Material; N,N-diethylcarbamodithioic acid [[diethylamino(sulfanylidene)methyl]thio] ester; THIOPEROXYDICARBONIC DIAMIDE ((H2N)C(S))(SUB 2) S(SUB 2), TETRAETHYL-; InChI=1/C10H20N2S4/c1-5-11(6-2)9(13)15-16-10(14)12(7-3)8-4/h5-8H2,1-4H; TETRAETHYLTHIOPEROXYDICARBONIC DIAMIDE ((((C(SUB 2)H(SUB 5))(SUB 2)N)C(S))(SUB 2)S(SUB 2))
Click to Show/Hide
|
||||
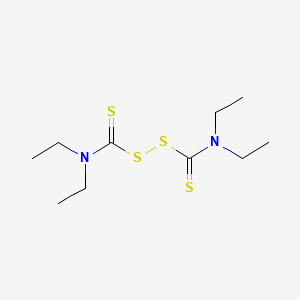
| Structure |
 |
||||
| Formula |
C10H20N2S4
|
||||
| IUPAC Name |
diethylcarbamothioylsulfanyl N,N-diethylcarbamodithioate
|
||||
| Canonical SMILES |
CCN(CC)C(=S)SSC(=S)N(CC)CC
|
||||
| InChI |
InChI=1S/C10H20N2S4/c1-5-11(6-2)9(13)15-16-10(14)12(7-3)8-4/h5-8H2,1-4H3
|
||||
| InChIKey |
AUZONCFQVSMFAP-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| 769-P cells | Renal cell carcinom | Homo sapiens | CVCL_1050 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MDA231-LM2-4175 cells | Breast adenocarcinoma | Homo sapiens | CVCL_5998 | ||
| In Vivo Model |
C57BL/6J male mice aged 8 weeks were purchased from Charles River Laboratories International, Inc., and housed in a specific pathogen-free animal facility. DMSO or DSF (21 mg/kg) was injected intraperitoneally into mice for 0.5 h, followed by ConA injection via the tail vein at 15 mg/kg. Mice were sacrificed at 24 h post ConA injection. Liver and blood samples were collected at this time point for H&E staining, IHC staining, and measurement of AST/ALT (Dian Diagnostics Co., Ltd).
Click to Show/Hide
|
||||
| Response regulation | Disulfiram (DSF) is conjugated to multiple cysteine residues in GPX4 and disrupts GPX4 interaction with HSC70, an adaptor protein for chaperone mediated autophagy, thus preventing GPX4 degradation induced by erastin. In addition, DSF ameliorates concanavalin A induced acute liver injury by suppressing ferroptosis in a mouse model. | ||||
