Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0108)
| Name |
Ketamine
|
||||
|---|---|---|---|---|---|
| Synonyms |
ketamine; dl-Ketamine; Ketaject; Special K; Ketalar; (+-)-Ketamine; CI 581 base; Calypsol; Ketaminum; CLSTA 20; 6740-88-1; (+/-)-Ketamine; narketan; Cetamina; Ketanest; Tekam; Ketalar base; 2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone; 2-(Methylamino)-2-(2-chlorophenyl)cyclohexanone; Ursotamin; Vetaket; Ketamine Base; KETAMINE HCL; Anaket v; 2-(o-Chlorophenyl)-2-(methylamino)-cyclohexanone; Special K [street name]; Clorketam 1000; Ketasol 100; Imalgene 1000; ketamina; NSC 70151; EINECS 229-804-1; NSC-70151; Ketamine (INN); UNII-690G0D6V8H; BRN 2216965; CHEBI:6121; 100477-72-3; DTXSID8023187; cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-; HSDB 2180; 690G0D6V8H; 2-(2-Chloro-phenyl)-2-methylamino-cyclohexanone; CHEMBL742; 2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one; (+/-)-2-(o-Chlorophenyl)-2-(methylamino)cyclohexanone; DTXCID703187; Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (+-)-; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (+-)-; KETAFOL COMPONENT KETAMINE; PMI-150; EC 229-804-1; 2-(o-Chlorophenyl)-2-(methylamino)cyclohexanone; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-; DEA No. 7285; NCGC00159480-02; NCGC00159480-03; Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (+/-)-; KETAMINE [INN]; Special K (street name); Ketamine [INN:BAN]; Ketaminum [INN-Latin]; Cetamina [INN-Spanish]; Ketoject; CAS-6740-88-1; Tekam (TN); Ketaminum (Latin); Ketolar (Salt/Mix); Vetalar (Salt/Mix); Kalipsol (Salt/Mix); Ketanest (Salt/Mix); KETAMINE [HSDB]; KETAMINE [MI]; KETAMINE [VANDF]; (.+/-.)-Ketamine; KETAMINE [WHO-DD]; Cyclohexanone, (.+-.)-; SCHEMBL16103; MLS001331674; DivK1c_000217; GTPL4233; (+/-)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone; SCHEMBL17084881; KBio1_000217; N01AX03; NINDS_000217; HMS2272G05; NSC70151; Tox21_111703; Tox21_111704; BDBM50044140; DB01221; IDI1_000217; SMR000238141; C07525; D08098; Q243547; 2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone #; J-505587; (.+/-.)-2-(O-Chlorophenyl)-2-(methylamino)cyclohexanone; 2-(2-Chloro-phenyl)-2-methylamino-cyclohexanone(Ketamine); Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)- (9CI); Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (+-)- (9CI); Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (.+/-.)-; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (.+/-.)-; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (+/-)- (8CI)
Click to Show/Hide
|
||||
| Structure |
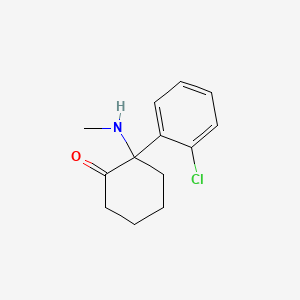 |
||||
| Formula |
C13H16ClNO
|
||||
| IUPAC Name |
2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one
|
||||
| Canonical SMILES |
CNC1(CCCCC1=O)C2=CC=CC=C2Cl
|
||||
| InChI |
InChI=1S/C13H16ClNO/c1-15-13(9-5-4-8-12(13)16)10-6-2-3-7-11(10)14/h2-3,6-7,15H,4-5,8-9H2,1H3
|
||||
| InChIKey |
YQEZLKZALYSWHR-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | |||
| Responsed Regulator | PVT1 (IncRNA) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 uL, 5 x 105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5 x width (mm)2 x length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Click to Show/Hide
|
||||
| Response regulation | LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of liver cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | |||
| Responsed Regulator | hsa-miR-214-3p (miRNA) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 uL, 5 x 105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5 x width (mm)2 x length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Click to Show/Hide
|
||||
| Response regulation | LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of liver cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Responsed Regulator | Histone acetyltransferase KAT5 (KAT5) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| Response regulation | The treatment of Ketamine induced the levels of MDA, lipid ROS, and Fe2+, while KAT5 or GPX4 overexpression could reverse this effect in breast cancer cells. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Ketamine may serve as a potential therapeutic strategy for breast cancer. | ||||
References
