Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0333)
| Name |
Curculigoside
|
||||
|---|---|---|---|---|---|
| Synonyms |
Curculigoside; 85643-19-2; curculigoside A; A6S7X76UM5; [5-hydroxy-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]methyl 2,6-dimethoxybenzoate; beta-D-Glucopyranoside, 2-(((2,6-dimethoxybenzoyl)oxy)methyl)-4-hydroxyphenyl; beta-D-Glucopyranoside, 2-[[(2,6-dimethoxybenzoyl)oxy]methyl]-4-hydroxyphenyl; Curculigoside,(S); UNII-A6S7X76UM5; CHEMBL258048; DTXSID30234896; HMS3886E06; HY-N0705; MFCD00800689; s5457; AKOS015897151; trihydroxy-6-(hydroxymethyl)tetrahydro; CCG-269418; AC-33958; AS-56484; C3504; CS-0009721; -2H-pyran-2-yloxy)benzyl 2,6-dimethoxybenzoate; Q-100862; Q5194662; 5-hydroxy-2-((2S,3R,4S,5S,6R)-3,4,5-; 2-(((2,6-DIMETHOXYBENZOYL)OXY)METHYL)-4-HYDROXYPHENYL .BETA.-D-GLUCOPYRANOSIDE; (5-Hydroxy-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)methyl 2,6-dimethoxybenzoate; [5-hydroxy-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]methyl2,6-dimethoxybenzoate
Click to Show/Hide
|
||||
| Structure |
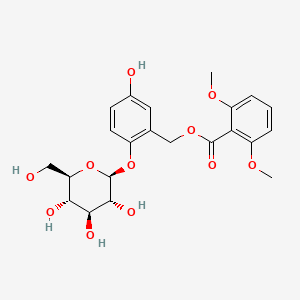 |
||||
| Formula |
C22H26O11
|
||||
| IUPAC Name |
[5-hydroxy-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]methyl 2,6-dimethoxybenzoate
|
||||
| Canonical SMILES |
COC1=C(C(=CC=C1)OC)C(=O)OCC2=C(C=CC(=C2)O)OC3C(C(C(C(O3)CO)O)O)O
|
||||
| InChI |
InChI=1S/C22H26O11/c1-29-14-4-3-5-15(30-2)17(14)21(28)31-10-11-8-12(24)6-7-13(11)32-22-20(27)19(26)18(25)16(9-23)33-22/h3-8,16,18-20,22-27H,9-10H2,1-2H3/t16-,18-,19+,20-,22-/m1/s1
|
||||
| InChIKey |
SJJRKHVKAXVFJQ-QKYBYQKWSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ulcerative colitis | ICD-11: DD71 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | IEC-6 cells | Normal | Rattus norvegicus | CVCL_0343 | |
| In Vivo Model |
Male C57BL/6J mice (8 weeks) were housed in a controlled condition at 25 , 45-55% humidity and 12 h light/dark cycle. All mice were randomly divided into five groups: Vehicle group, mice received dextran sulfate sodium (DSS group), DSS mice received ferrostatin-1 (DSS + Fer-1 group), DSS mice received low dose of CUR (DSS + CUR-L group), and DSS mice received high dose of CUR (DSS + CUR-H group). In the experiments, 3% DSS (D122347, Aladdin, Shanghai, China) in drinking water for 7 days was prepared to induce UC models. Mice in DSS + Fer-1 group were intraperitoneally injected with 5 mg/kg Fer-1 (S7243, Selleck, Shanghai, China) every two days from the day before DSS induction. In addition, mice in DSS + CUR-L or DSS + CUR-H group received intragastric administration with CUR (HY-N0705, Med Chem Express, Shanghai, China) at 50 mg/kg or 100 mg/kg once a day for 7 days during DSS administration.
Click to Show/Hide
|
||||
| Response regulation | Curculigoside (CUR) could increase the selenium sensitivity and promote GPX4 transcription level in IEC-6 cells. Knockdown of GPX4 significantly blocked the protective effects of CUR on cell death, GSH and MDA contents as well as LDH activity in ferroptotic IEC-6 cells. Taken together, CUR protects against ferroptosis in ulcerative colitis (UC) by the induction of GPX4, which presents a potential agent for UC treatment. | ||||
