Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0144)
| Name |
Cucurbitacin B
|
||||
|---|---|---|---|---|---|
| Synonyms |
Cucurbitacin B; 6199-67-3; Amarine; Datisca principle B; DATISCACIN; Datiscn Principle B; (+)-Cucurbitacin B; Cucurbitacine B; 1,2-Dihydro-alpha-elaterin; UNII-0115W5MABF; 0115W5MABF; CHEBI:3941; CUCURBITACIN R - DATISCA PRINCIPLE B; HSDB 3476; NSC49451; NSC 49451; NSC-49451; NSC 144154; NSC-144154; Cucurbitacin B hydrate; (R,E)-6-((2S,8S,9R,10R,13R,14S,16R,17R)-2,16-dihydroxy-4,4,9,13,14-pentamethyl-3,11-dioxo-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-6-hydroxy-2-methyl-5-oxohept-3-en-2-yl acetate; [(E,6R)-6-[(2S,8S,9R,10R,13R,14S,16R,17R)-2,16-dihydroxy-4,4,9,13,14-pentamethyl-3,11-dioxo-2,7,8,10,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-hydroxy-2-methyl-5-oxohept-3-en-2-yl] acetate; C32H46O8; Cucurbitacine (B); Cucurbitacin-B; Cuc B; 25-(Acetyloxy)-2,16,20-trihydroxy-9-methyl-19-norlanosta-5,23-diene-3,11,22-trione; 19-Nor-9beta,10alpha-lanosta-5,23-diene-3,11,22-trione, 2beta,16alpha,20,25-tetrahydroxy-9-methyl-, 25-acetate; 19-Norlanosta-5,23-diene-3,11,22-trione, 25-(acetyloxy)-2,16,20-trihydroxy-9-methyl-, (2.beta.,9.beta.,10.alpha.,16.alpha.,23E)-; 19-Norlanosta-5,23-diene-3,11,22-trione, 25-(acetyloxy)-2,16,20-trihydroxy-9-methyl-, (2beta,9beta,10alpha,16alpha,23E)-; MLS002702988; 19-nor-9.beta.,23-diene-3,11,22-trione, 9-methyl-2.beta.,16.alpha.,20,25-tetrahydroxy-, 25-acetate; 19-Norlanosta-5,11,22-trione, 25-(acetyloxy)-2,16,20-trihydroxy-9-methyl-, (2.beta.,9.beta.,10.alpha.,16.alpha.,23E)-; CUCURBITACIN B [MI]; CUCURBITACIN B [INCI]; SCHEMBL231815; CHEMBL508185; GTPL12432; DTXSID501316196; 19-Nor-9-beta,10-alpha-lanosta-5,23-diene-3,11,22-trione, 2-beta,16-alpha,20,25-tetrahydroxy-9-methyl-, 25-acetate; HY-N0416; LMST01010104; MFCD07778083; NSC144154; s8165; AKOS015897085; 1,2-DIHYDRO-.ALPHA.-ELATERIN; CCG-270043; CS-3816; AC-34283; Cucurbitacin B hydrate, >=97% (HPLC); C3442; C08794; A924078; Q-100715; Q27106259; (2.BETA.,9.BETA.,10.ALPHA.,16.ALPHA.,23E)-25-(ACETYLOXY)-2,16,20-TRIHYDROXY-9-METHYL-19-NORLANOSTA-5,23-DIENE-3,11,22-TRIONE; (2beta,9beta,10alpha,16alpha,23E)-25-(acetyloxy)-2,16,20-trihydroxy-9-methyl-19-Norlanosta-5,23-diene-3,11,22-trione; (2S,4R,23E)-2,16beta,20-trihydroxy-9beta,10,14-trimethyl-1,11,22-trioxo-4,9-cyclo-9,10-secocholesta-5,23-dien-25-yl acetate; (3E,6R)-6-[(1R,2R,4S,10S,11S,13R,14R,15R)-4,13-dihydroxy-1,6,6,11,15-pentamethyl-5,17-dioxotetracyclo[8.7.0.0?,?.0??,??]heptadec-7-en-14-yl]-6-hydroxy-2-methyl-5-oxohept-3-en-2-yl acetate; 19-Norlanosta-5,11,22-trione, 25-(acetyloxy)-2,16,20-trihydroxy-9-methyl-, [2.beta.,.gamma.,.beta.,10.alpha.,16.alpha.]-; 2beta,16alpha,20,25-tetrahydroxy-9-methyl-19-nor-9beta,10alpha-lanosta-5,23-diene-3,11,22-trione, 25-acetate; 2beta,16alpha,20,25-tetrahydroxy-9-methyl-3,11,22-trioxo-19-nor-9beta,10alpha-lanosta-5,23-dien-25-yl acetate
Click to Show/Hide
|
||||
| Status |
Phase 3
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
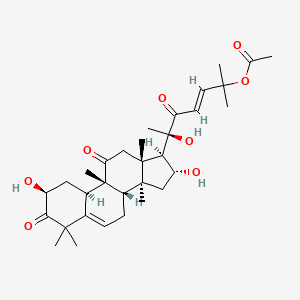 |
||||
| Formula |
C32H46O8
|
||||
| IUPAC Name |
[(E,6R)-6-[(2S,8S,9R,10R,13R,14S,16R,17R)-2,16-dihydroxy-4,4,9,13,14-pentamethyl-3,11-dioxo-2,7,8,10,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-hydroxy-2-methyl-5-oxohept-3-en-2-yl] acetate
|
||||
| Canonical SMILES |
CC(=O)OC(C)(C)C=CC(=O)C(C)(C1C(CC2(C1(CC(=O)C3(C2CC=C4C3CC(C(=O)C4(C)C)O)C)C)C)O)O
|
||||
| InChI |
InChI=1S/C32H46O8/c1-17(33)40-27(2,3)13-12-23(36)32(9,39)25-21(35)15-29(6)22-11-10-18-19(14-20(34)26(38)28(18,4)5)31(22,8)24(37)16-30(25,29)7/h10,12-13,19-22,25,34-35,39H,11,14-16H2,1-9H3/b13-12+/t19-,20+,21-,22+,25+,29+,30-,31+,32+/m1/s1
|
||||
| InChIKey |
IXQKXEUSCPEQRD-DKRGWESNSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Nasopharyngeal cancer | ICD-11: 2B6B | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| CNE1 cells | Nasopharyngeal carcinoma | Homo sapiens | CVCL_6888 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| In Vivo Model |
The animal experiment was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University. BALB/c nude mice (5 weeks old, female, Guangdong Medical Laboratory Animal Centre, China) were used for animal experiments. Approximately 4 million CNE1 cells were injected subcutaneously into the right flank of each mouse. Palpable solid tumours developed within a month after tumour cell inoculation, and mice were randomly allocated to four different groups (five mice /group) as follows: control (PBS) group, CuB treatment groups [0.5 mg/kg (low-dose) group and 1 mg/kg (high-dose) group] and gemcitabine (GEM, 25 mg/kg) group. Mice received intraperitoneal injections of PBS, CuB, and gemcitabine 3 times weekly.
Click to Show/Hide
|
||||
| Response regulation | Cucurbitacin B caused intracellular accumulation of iron ions and depletion of glutathione. Detailed molecular mechanism investigation confirmed that CuB both induced widespread lipid peroxidation and downregulated the expression of GPX4, ultimately initiating a multipronged mechanism of ferroptosis. The study highlighted the therapeutic potential of CuB as a ferroptosis-inducing agent for nasopharyngeal cancer. | ||||
