Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0339)
| Name |
[4-[Bis(4-chlorophenyl)methyl]piperazin-1-yl]-(5-methyl-4-nitro-1,2-oxazol-3-yl)methanone
|
||||
|---|---|---|---|---|---|
| Synonyms |
1360705-96-9; ML-210; [4-[bis(4-chlorophenyl)methyl]piperazin-1-yl]-(5-methyl-4-nitro-1,2-oxazol-3-yl)methanone; ML 210; (4-(bis(4-chlorophenyl)methyl)piperazin-1-yl)(5-methyl-4-nitroisoxazol-3-yl)methanone; ML210; CHEMBL1951048; CID 49766530; BRD7528; BRD-7528; TL_HRAS26; MLS003265661; SCHEMBL21965947; CHEBI:92034; HMS3874L03; BCP29439; EX-A3516; BDBM50547193; MFCD22666407; NCGC00386679-01; NCGC00386679-02; AC-36416; BM170835; BS-51618; SMR001941104; HY-100003; ML 210, >=98% (HPLC); CS-0017911; S0788; D83838; BRD-K01877528-001-01-6; BRD-K01877528-001-02-4; BRD-K01877528-001-03-2; BRD-K01877528-001-04-0; BRD-K01877528-001-07-3; BRD-K01877528-001-08-1; BRD-K01877528-001-09-9; BRD-K01877528-001-11-5; Q27163827; [4-[Bis(4-chlorophenyl)methyl]-1-piperazinyl](5-methyl-4-nitro-3-isoxazolyl)methanone; [4-[bis(4-chlorophenyl)methyl]-1-piperazinyl]-(5-methyl-4-nitro-3-isoxazolyl)methanone; CID 49766530; CID-49766530; CID49766530; ML-210; ML 210
Click to Show/Hide
|
||||
| Structure |
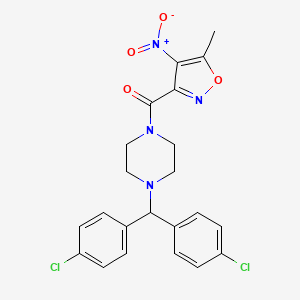 |
||||
| Formula |
C22H20Cl2N4O4
|
||||
| IUPAC Name |
[4-[bis(4-chlorophenyl)methyl]piperazin-1-yl]-(5-methyl-4-nitro-1,2-oxazol-3-yl)methanone
|
||||
| Canonical SMILES |
CC1=C(C(=NO1)C(=O)N2CCN(CC2)C(C3=CC=C(C=C3)Cl)C4=CC=C(C=C4)Cl)[N+](=O)[O-]
|
||||
| InChI |
InChI=1S/C22H20Cl2N4O4/c1-14-20(28(30)31)19(25-32-14)22(29)27-12-10-26(11-13-27)21(15-2-6-17(23)7-3-15)16-4-8-18(24)9-5-16/h2-9,21H,10-13H2,1H3
|
||||
| InChIKey |
VIBHJPDPEVVDTB-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Melanoma | ICD-11: 2C30 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | LOX-IMVI cells | Melanoma | Homo sapiens | CVCL_1381 |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| HEK293-EBNA1-6E cells | Normal | Homo sapiens | CVCL_HF20 | |
| CJM cells | Melanoma | Homo sapiens | CVCL_U797 | |
| WM88 cells | Melanoma | Homo sapiens | CVCL_6805 | |
| KP-4 cells | Pancreatic carcinoma | Homo sapiens | CVCL_1338 | |
| HCC4006 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1269 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-2 cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_0235 | |
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | |
| MC-38 cells | Colon adenocarcinoma | Homo sapiens | CVCL_B288 | |
| Response regulation | ML210 is a prodrug that is converted in cells into a nitrile-oxide electrophile that covalently inhibits GPX4 with remarkable proteome-wide selectivity in melanoma. | |||
