Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10056)
| Target Name | Serotransferrin (TF) | ||||
|---|---|---|---|---|---|
| Synonyms |
Beta-1 metal-binding globulin; Siderophilin
Click to Show/Hide
|
||||
| Gene Name | TF | ||||
| Sequence |
MRLAVGALLVCAVLGLCLAVPDKTVRWCAVSEHEATKCQSFRDHMKSVIPSDGPSVACVK
KASYLDCIRAIAANEADAVTLDAGLVYDAYLAPNNLKPVVAEFYGSKEDPQTFYYAVAVV KKDSGFQMNQLRGKKSCHTGLGRSAGWNIPIGLLYCDLPEPRKPLEKAVANFFSGSCAPC ADGTDFPQLCQLCPGCGCSTLNQYFGYSGAFKCLKDGAGDVAFVKHSTIFENLANKADRD QYELLCLDNTRKPVDEYKDCHLAQVPSHTVVARSMGGKEDLIWELLNQAQEHFGKDKSKE FQLFSSPHGKDLLFKDSAHGFLKVPPRMDAKMYLGYEYVTAIRNLREGTCPEAPTDECKP VKWCALSHHERLKCDEWSVNSVGKIECVSAETTEDCIAKIMNGEADAMSLDGGFVYIAGK CGLVPVLAENYNKSDNCEDTPEAGYFAVAVVKKSASDLTWDNLKGKKSCHTAVGRTAGWN IPMGLLYNKINHCRFDEFFSEGCAPGSKKDSSLCKLCMGSGLNLCEPNNKEGYYGYTGAF RCLVEKGDVAFVKHQTVPQNTGGKNPDPWAKNLNEKDYELLCLDGTRKPVEEYANCHLAR APNHAVVTRKDKEACVHKILRQQQHLFGSNVTDCSGNFCLFRSETKDLLFRDDTVCLAKL HDRNTYEKYLGEEYVKAVGNLRKCSTSSLLEACTFRRP Click to Show/Hide
|
||||
| Family | Transferrin family | ||||
| Function |
Transferrins are iron binding transport proteins which can bind two Fe(3+) ions in association with the binding of an anion, usually bicarbonate. It is responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation.; (Microbial infection) Serves as an iron source for Neisseria species, which capture the protein and extract its iron for their own use.
Click to Show/Hide
|
||||
| Gene ID | 7018 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
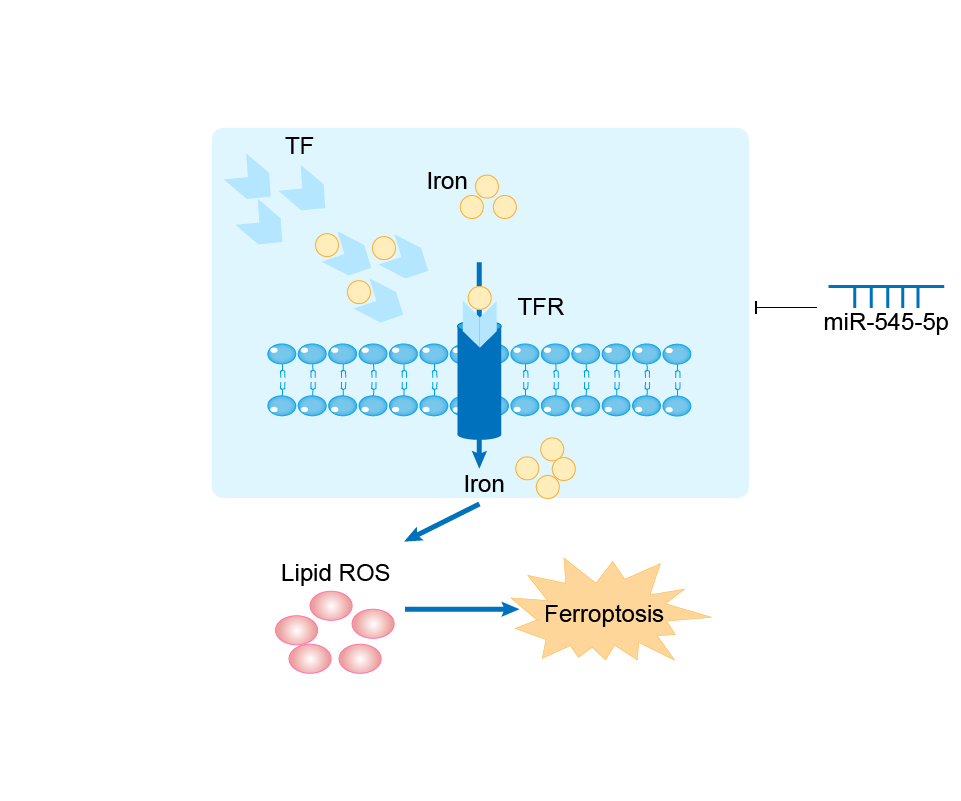
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
TF can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Mitogen-activated protein kinase 1 (MAPK1)
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Simvastatin | Investigative | ||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 |
| Response Description | Simvastatin has the potential to be a targeted drug for endometrial cancer (EC) treatment. Besides, the inhibition to the RAS/MAPK signaling pathway allows simvastatin to induce ferroptosis through up-regulating the level of ROS, MDA, Fe2+, and TRF1 (TF) and reducing the level of GSH, SLC7A11, and FPN in cells. | |||
Sterol regulatory element-binding protein 2
Melanoma [ICD-11: 2C30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
hCTCs (Human circulating tumor cells) | ||||
| IGR-37 cells | Melanoma | Homo sapiens | CVCL_2075 | ||
| SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 | ||
| GAK cells | Vulvar melanoma | Homo sapiens | CVCL_1225 | ||
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| In Vivo Model |
For primary tumorigenesis assays, NOD-scid Il2rg-/-mice (6-8 weeks old, female) were injected subcutaneously in the left flank with cultured CTCs, and tumors were harvested when they reached 2 centimeters in diameter.
Click to Show/Hide
|
||||
| Response Description | The lipogenesis regulator SREBP2 directly induces transcription of the iron carrier Transferrin (TF), reducing intracellular iron pools, reactive oxygen species, and lipid peroxidation, thereby conferring resistance to inducers of ferroptosis. SREBP2-driven iron homeostatic pathways contribute to cancer progression, drug resistance, and metastasis in melanoma cancers. | ||||
Peroxisome proliferator-activated receptor alpha (PPARA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hepa 1-6 cells | Hepatocellular carcinoma | Mus musculus | CVCL_0327 | |
| In Vivo Model |
C57BL/6J SPF mice were purchased from Huazhong Agricultural University Experimental Animal Center. Mice were given tertian intraperitoneal injections of either PBS (control) or dextriferron (500 mg/kg body weight) for 2 weeks and then sacrificed. Mice were given a daily intraperitoneal injection of either vehicle or ferrostatin-1 (Fer1, 1 mg/kg body weight) for 3 weeks before sacrificed.
Click to Show/Hide
|
||||
| Response Description | PPARa activation alleviates iron overload-induced ferroptosis in mouse livers through Gpx4 and TRF (TF), suggesting that PPAR may be a promising therapeutic target for drug discovery in ferroptosis-related tissue injuries in Hepatocellular carcinomaHepatocellular carcinoma. | ||||
hsa-miR-545-3p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NCM460 cells | Normal | Homo sapiens | CVCL_0460 | |
| HT-29 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| In Vivo Model |
5-week-old immunodeficient nude mice (male, weight, 16-20 g, n = 40 mice for each group) were purchased from Cyagen bio. Co. (Beijing, China). Before experiments, the mice were adapted to the breeding environment for two weeks. All mice were maintained at a 12 h/12 h light/dark cycle with free access to water and food. A total of 5 x 106 HT-29 or HCT-116 cells were suspended in 100 uL PBS and injected subcutaneously into the right posterior flanks of nude mice. After three weeks, the mice were killed and the tumor xenografts were dissected, weighed and fixed in 10% buffered formaldehyde for further IHC analysis.
Click to Show/Hide
|
||||
| Response Description | MiR-545 inhibits ferroptosis in colorectal cancer cells by inhibiting TF. TF overexpression blocked miR-545-induced changes in ROS, MDA, and Fe2+ levels in HT-29 and HCT-116 cells, thereby inducing CRC cell death. | ||||
Mitogen-activated protein kinase 1 (MAPK1)
Simvastatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Corpus uteri cancer [ICD-11: 2C76] | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 |
| Response Description | Simvastatin has the potential to be a targeted drug for endometrial cancer (EC) treatment. Besides, the inhibition to the RAS/MAPK signaling pathway allows simvastatin to induce ferroptosis through up-regulating the level of ROS, MDA, Fe2+, and TRF1 (TF) and reducing the level of GSH, SLC7A11, and FPN in cells. | |||
References
