Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10059)
| Target Name | Transferrin receptor protein 2 (TFR2) | ||||
|---|---|---|---|---|---|
| Gene Name | TFR2 | ||||
| Sequence |
MERLWGLFQRAQQLSPRSSQTVYQRVEGPRKGHLEEEEEDGEEGAETLAHFCPMELRGPE
PLGSRPRQPNLIPWAAAGRRAAPYLVLTALLIFTGAFLLGYVAFRGSCQACGDSVLVVSE DVNYEPDLDFHQGRLYWSDLQAMFLQFLGEGRLEDTIRQTSLRERVAGSAGMAALTQDIR AALSRQKLDHVWTDTHYVGLQFPDPAHPNTLHWVDEAGKVGEQLPLEDPDVYCPYSAIGN VTGELVYAHYGRPEDLQDLRARGVDPVGRLLLVRVGVISFAQKVTNAQDFGAQGVLIYPE PADFSQDPPKPSLSSQQAVYGHVHLGTGDPYTPGFPSFNQTQFPPVASSGLPSIPAQPIS ADIASRLLRKLKGPVAPQEWQGSLLGSPYHLGPGPRLRLVVNNHRTSTPINNIFGCIEGR SEPDHYVVIGAQRDAWGPGAAKSAVGTAILLELVRTFSSMVSNGFRPRRSLLFISWDGGD FGSVGSTEWLEGYLSVLHLKAVVYVSLDNAVLGDDKFHAKTSPLLTSLIESVLKQVDSPN HSGQTLYEQVVFTNPSWDAEVIRPLPMDSSAYSFTAFVGVPAVEFSFMEDDQAYPFLHTK EDTYENLHKVLQGRLPAVAQAVAQLAGQLLIRLSHDRLLPLDFGRYGDVVLRHIGNLNEF SGDLKARGLTLQWVYSARGDYIRAAEKLRQEIYSSEERDERLTRMYNVRIMRVEFYFLSQ YVSPADSPFRHIFMGRGDHTLGALLDHLRLLRSNSSGTPGATSSTGFQESRFRRQLALLT WTLQGAANALSGDVWNIDNNF Click to Show/Hide
|
||||
| Family | Peptidase M28 family | ||||
| Function |
Mediates cellular uptake of transferrin-bound iron in a non- iron dependent manner. May be involved in iron metabolism, hepatocyte function and erythrocyte differentiation.
Click to Show/Hide
|
||||
| Gene ID | 7036 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
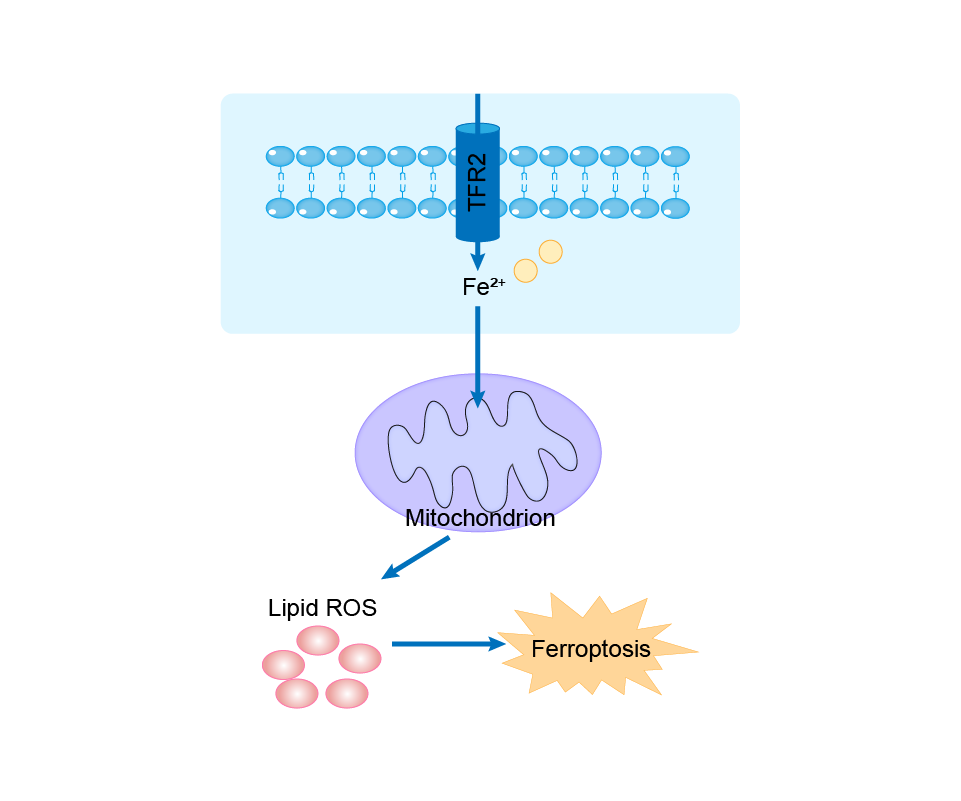
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
TFR2 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
hsa-miR-486-3p (miRNA)
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hNPCs (Human nucleus pulposus cells) | |||
| Response Description | Circ-STC2 and TFR2 expressions were up-regulated in intervertebral disc degeneration (IDD) tissues, and miR-486-3p expression was down-regulated. Circ-STC2 inhibits the cell viability, induced the ferroptosis of the TBHP treated NPCs via targeting miR-486-3p/TFR2 axis. | |||
Circ_STC2 (circRNA)
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hNPCs (Human nucleus pulposus cells) | |||
| Response Description | Circ-STC2 and TFR2 expressions were up-regulated in intervertebral disc degeneration (IDD) tissues, and miR-486-3p expression was down-regulated. Circ-STC2 inhibits the cell viability, induced the ferroptosis of the TBHP treated NPCs via targeting miR-486-3p/TFR2 axis. | |||
Unspecific Regulator
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| Response Description | Overexpression of TFR2 promoted the production of reactive oxygen species and lipid peroxidation in glioma cells, thereby further promoting ferroptosis. In conclusion, TFR2 induced ferroptosis and enhanced TMZ sensitivity in gliomas. | |||
References
