Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10027)
| Target Name | Aspartate aminotransferase, cytoplasmic (GOT1) | ||||
|---|---|---|---|---|---|
| Synonyms |
Cysteine aminotransferase, cytoplasmic; Cysteine transaminase, cytoplasmic; Glutamate oxaloacetate transaminase 1; Transaminase A
Click to Show/Hide
|
||||
| Gene Name | GOT1 | ||||
| Sequence |
MAPPSVFAEVPQAQPVLVFKLTADFREDPDPRKVNLGVGAYRTDDCHPWVLPVVKKVEQK
IANDNSLNHEYLPILGLAEFRSCASRLALGDDSPALKEKRVGGVQSLGGTGALRIGADFL ARWYNGTNNKNTPVYVSSPTWENHNAVFSAAGFKDIRSYRYWDAEKRGLDLQGFLNDLEN APEFSIVVLHACAHNPTGIDPTPEQWKQIASVMKHRFLFPFFDSAYQGFASGNLERDAWA IRYFVSEGFEFFCAQSFSKNFGLYNERVGNLTVVGKEPESILQVLSQMEKIVRITWSNPP AQGARIVASTLSNPELFEEWTGNVKTMADRILTMRSELRARLEALKTPGTWNHITDQIGM FSFTGLNPKQVEYLVNEKHIYLLPSGRINVSGLTTKNLDYVATSIHEAVTKIQ Click to Show/Hide
|
||||
| Family | Class-I pyridoxal-phosphate-dependent aminotransferase family | ||||
| Function |
Biosynthesis of L-glutamate from L-aspartate or L-cysteine. Important regulator of levels of glutamate, the major excitatory neurotransmitter of the vertebrate central nervous system. Acts as a scavenger of glutamate in brain neuroprotection. The aspartate aminotransferase activity is involved in hepatic glucose synthesis during development and in adipocyte glyceroneogenesis. Using L-cysteine as substrate, regulates levels of mercaptopyruvate, an important source of hydrogen sulfide. Mercaptopyruvate is converted into H(2)S via the action of 3-mercaptopyruvate sulfurtransferase (3MST). Hydrogen sulfide is an important synaptic modulator and neuroprotectant in the brain. In addition, catalyzes (2S)-2- aminobutanoate, a by-product in the cysteine biosynthesis pathway.
Click to Show/Hide
|
||||
| Gene ID | 2805 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
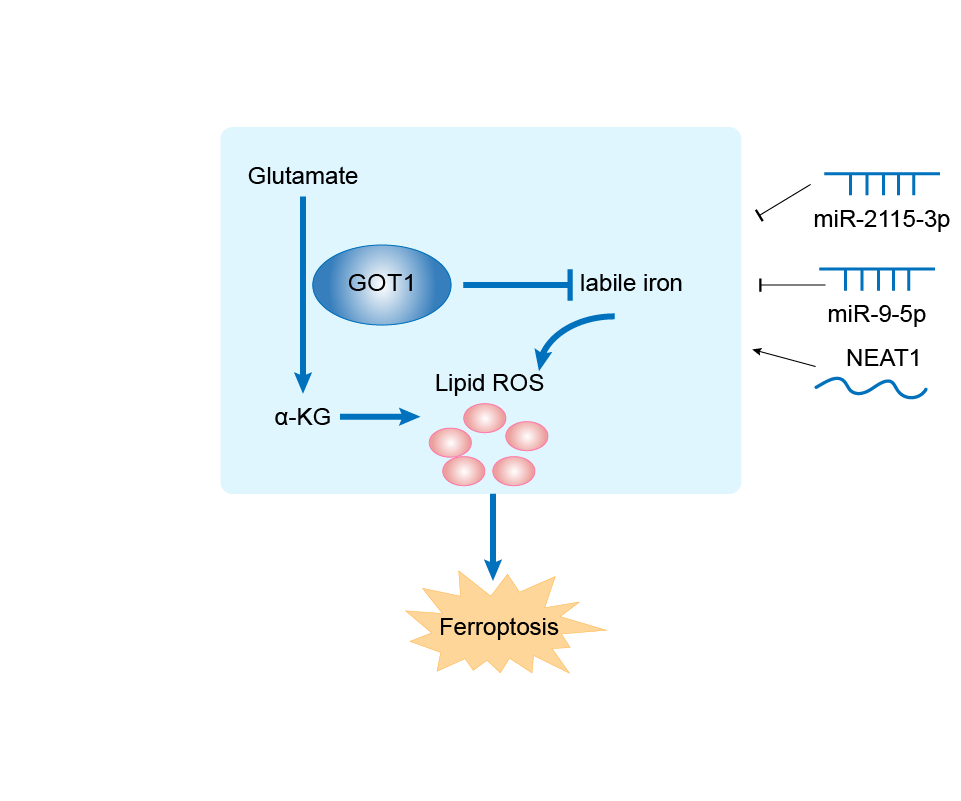
GOT1 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
NEAT1 (IncRNA)
Sepsis [ICD-11: 1G40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Thirty specific-pathogen-free (SPF) male C57BL/6 rats at 8 weeks weighing 200-250 g were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). The rats were housed under room temperatures (25 ± 2 and 12-h light/dark cycle) and given water and food ad libitum before the trial. Then, they were randomly assigned into the control group (n = 10), model group (n = 10), and model + miR-9-5p angomir group (n = 10). All animal procedures were performed under the Care and Use of Laboratory Animals guidelines and approved by the Guangdong Academy of Medical Sciences. According to a previous study, sepsis was induced by the cecal ligation and puncture (CLP) method. Briefly, we anesthetized the rats with 5% chloral hydrate (0.6 ml/100 g body weight) and made a 1.5 cm midline incision on the anterior abdomen to expose the cecum. Then, the cecum was ligated at 30%, punctured twice with a No.4 surgical needle to extrude the fecal content. Finally, 1 ml of normal saline was used for resuscitation. The rats in miR-9-5p angomir group were injected with 100 ng/ul miR-9-5p angomir (RiboBio, Guangzhou, China) into caudal vein (model + miR-9-5p angomir). The rats in the control group experienced the same procedure without ligation and puncture.
Click to Show/Hide
|
||||
| Response Description | NEAT1 functions as a ceRNA for miR-9-5p to facilitate TFRC and GOT1 expression. Overexpression of NEAT1 enhanced ferroptosis stress in bEnd.3 cells. Increased miR-9-5p alleviated sepsis-induced ferroptosis by suppressing the expression of TFRC and GOT1 both in vivo and in vitro. | ||||
hsa-miR-9-5p (miRNA)
Sepsis [ICD-11: 1G40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Thirty specific-pathogen-free (SPF) male C57BL/6 rats at 8 weeks weighing 200-250 g were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). The rats were housed under room temperatures (25 ± 2 and 12-h light/dark cycle) and given water and food ad libitum before the trial. Then, they were randomly assigned into the control group (n = 10), model group (n = 10), and model + miR-9-5p angomir group (n = 10). All animal procedures were performed under the Care and Use of Laboratory Animals guidelines and approved by the Guangdong Academy of Medical Sciences. According to a previous study, sepsis was induced by the cecal ligation and puncture (CLP) method. Briefly, we anesthetized the rats with 5% chloral hydrate (0.6 ml/100 g body weight) and made a 1.5 cm midline incision on the anterior abdomen to expose the cecum. Then, the cecum was ligated at 30%, punctured twice with a No.4 surgical needle to extrude the fecal content. Finally, 1 ml of normal saline was used for resuscitation. The rats in miR-9-5p angomir group were injected with 100 ng/ul miR-9-5p angomir (RiboBio, Guangzhou, China) into caudal vein (model + miR-9-5p angomir). The rats in the control group experienced the same procedure without ligation and puncture.
Click to Show/Hide
|
||||
| Response Description | NEAT1 functions as a ceRNA for miR-9-5p to facilitate TFRC and GOT1 expression. Overexpression of NEAT1 enhanced ferroptosis stress in bEnd.3 cells. Increased miR-9-5p alleviated sepsis-induced ferroptosis by suppressing the expression of TFRC and GOT1 both in vivo and in vitro. | ||||
hsa-miR-2115-3p (miRNA)
Preeclampsia [ICD-11: JA24]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HTR-8/SVneo cells | Normal | Homo sapiens | CVCL_7162 | |
| In Vivo Model |
Pregnant rats were randomly divided into 3 groups on gestational day 13, with 8 in each group: Sham group, PE group, and PE + ferrostatin-1 (FI) group. As previously mentioned, on gestational day 14, rats in the PE and PE + FI groups were subjected to reduced uterine perfusion pressure (RUPP) surgery to establish the PE model. Briefly, pregnant rats were anesthetized, sterilized with iodophor, and then opened. A contractile silver clip (0.203 mm) was placed on the aorta above the iliac bifurcation, as well as the bilateral uterine arcades at the end of the ovary were reduced with restrictive clips (0.100 mm) to the ovarian collaterals of the uterus. The rats in the Sham group were operated in a manner similar to the RUPP rats but were not clipped. On a gestational day 14, the mini-pumps were also inserted into the rat's peritoneum. The mini-pump in each rat of the PE + FI group was used to deliver FI at a dose of 10 mol/kg/d for 5 d. Systolic and diastolic blood pressure (SBP and DBP) were measured on gestational days 14, 16, and 19 using catheters inserted into the carotid artery and jugular vein. On a gestational day 19, urine, blood, and placenta of rats were collected for subsequent experiments. Urine protein content was detected using a commercial kit (C035-2, Nanjing Jiancheng, China). Plasma sFlt-1 content was detected by a commercial kit (ml059017, Mlbio, China). The remaining samples were stored at -80 .
Click to Show/Hide
|
||||
| Response Description | miR-2115-3p might interact with the GOT1 mRNA to downregulate its expression, further inhibiting the hypoxia-promoted ferroptosis in a preeclampsia model. | ||||
References
