Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0123)
| Name |
Everolimus
|
||||
|---|---|---|---|---|---|
| Synonyms |
001, RAD; 40-O-(2-hydroxyethyl)-rapamycin; 40-O-(2-Hydroxyethyl)rapamycin; Afinitor; Certican; Everolimus; RAD; RAD 001; RAD, SDZ; RAD001; SDZ RAD; SDZ-RAD; Zortress; 159351-69-6; Votubia; 42-O-(2-Hydroxyethyl)rapamycin; RAD-001; Afinitor Disperz; Everolimus [USAN]; CHEBI:68478; Rapamycin, 42-O-(2-hydroxyethyl)-; 9HW64Q8G6G; DTXSID0040599; RAD 666; RAD-666; Everolimus (INN); XIENCE V; NCGC00167512-01; Everolimus (RAD001); EVEROLIMUS [INN]; UNII-9HW64Q8G6G; (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-{(2R)-1-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone; Everolimus [USAN:INN:BAN]; (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-Dihydroxy-12-((1R)-2-((1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo(30.3.1.0(sup 4,9))hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone; (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-Dihydroxy-12-((1R)-2-((1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo(30.3.1.04,9)hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone; (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,35R)-1,18-dihydroxy-12-{(2R)-1-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.0(4,9)]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone; (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-((1R)-2-((1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone; NVP-RAD-001; RAD-001C; everolimusum; NSC733504; EVE - Everolimus; SDZRAD; EVEROLIMUS [MI]; Everolimus - RAD001; EVEROLIMUS [JAN]; EVEROLIMUS [VANDF]; EVEROLIMUS [MART.]; SCHEMBL4378; EVEROLIMUS [USP-RS]; EVEROLIMUS [WHO-DD]; NVP-RAD001; EVEROLIMUS [EMA EPAR]; Everolimus, analytical standard; GTPL5889; CHEMBL1908360; DTXCID8020599; EVEROLIMUS [ORANGE BOOK]; HSDB 8255; EVEROLIMUS [EP MONOGRAPH]; Everolimus; RAD001; SDZ-RAD; HKVAMNSJSFKALM-GKUWKFKPSA-N; 42-O-(2-Hydroxyethyl)-rapamycin; EX-A2057; Tox21_112510; BDBM50088378; AKOS015850977; CS-0064; DB01590; AS-16971; HY-10218; LS-143292; CAS-159351-69-6; Q421052; Q-101413; BRD-K13514097-001-01-2; BRD-K13514097-001-05-3; dihydroxy-[(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxy-cyclohexyl]-1-methyl-ethyl]-dimethoxy-hexamethyl-[?]pentone
Click to Show/Hide
|
||||
| Structure |
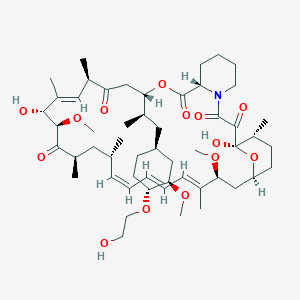 |
||||
|
3D MOL
|
|||||
| Formula |
C53H83NO14
|
||||
| IUPAC Name |
(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
|
||||
| Canonical SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OCCO)C)C)O)OC)C)C)C)OC
|
||||
| InChI |
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
|
||||
| InChIKey |
HKVAMNSJSFKALM-GKUWKFKPSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Stearoyl-CoA desaturase (SCD)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | ||
| Responsed Regulator | Serine/threonine-protein kinase mTOR (MTOR) | Suppressor | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | BON-1 cells | Pancreatic serotonin-producing neuroendocrine tumor | Homo sapiens | CVCL_3985 |
| QGP-1 cells | Pancreatic somatostatinoma | Homo sapiens | CVCL_3143 | |
| Response regulation | The negative correlation between MEN1 and SCD1 is further verified in clinical specimens. Furthermore, BON-1 and QGP-1 cells with MEN1 overexpression are more sensitive to everolimus, a widely used drug in pancreatic neuroendocrine tumors (pNETs) that targets mTOR signaling. | |||
Unspecific Target
| In total 2 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Responsed Disease | Hereditary Leiomyomatosis | ICD-11: 2C90 | ||
| Responsed Regulator | Serine/threonine-protein kinase mTOR (MTOR) | Suppressor | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Glutathione metabolism | hsa00480 | |||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| HEK293 cells | Normal | Homo sapiens | CVCL_0045 | |
| Response regulation | Everolimus and RSL3/Erastin could synergistically inhibit the viability and induce ferroptosis in Renal cell carcinoma cells. Mechanistically, the inhibition of the mTOR-4EBP1 axis was found to be essential for the synergistic effects of Everolimus and RSL3/Erastin. Everolimus in combination with RSL3/Erastin is a promising therapeutic option for RCC treatment. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Responsed Disease | Hereditary Leiomyomatosis | ICD-11: 2C90 | ||
| Responsed Regulator | Eukaryotic translation initiation factor 4E (EIF4E) | Suppressor | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Glutathione metabolism | hsa00480 | |||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| HEK293 cells | Normal | Homo sapiens | CVCL_0045 | |
| Response regulation | Everolimus and RSL3/Erastin could synergistically inhibit the viability and induce ferroptosis in Renal cell carcinoma cells. Mechanistically, the inhibition of the mTOR-4EBP1 axis was found to be essential for the synergistic effects of Everolimus and RSL3/Erastin. Everolimus in combination with RSL3/Erastin is a promising therapeutic option for RCC treatment. | |||
References
